| Molbank 2003, M322 |
(±)-2-tButyl-7-methoxy-1-oxaspiro[4,5]deca-6,9-diene-8-one
Guy L. Plourde
University of Northern British Columbia, Department of Chemistry, 3333 University Way, Prince George, British Columbia, Canada, V2N 4Z9
Tel: 250-960-6694, Fax: 250-960-5545, E-mail: [email protected]
Received: 7 July 2002 / Accepted: 15 October 2002 / Published: 6 April 2003
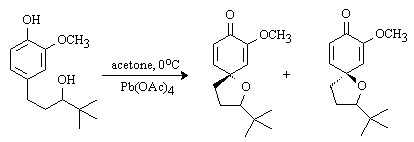
The discussion and purpose for the synthesis of this compound has been reported elsewhere [1]. To a cold (0°C) solution of (±)-1-(4-hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanol (216 mg, 0.91 mmol) in acetone (25 mL) was added in one portion Pb(OAc)4 (1.3 g, 2.9 mmol, 3.1 eq). The resulting orange mixture was stirred at 0°C for 2 h. The precipitate was filtered through celite and ethylene glycol (10 drops) was added. The solution was stirred at room temperature for 20 h and filtered through celite. The solvent was evaporated in vacuo to afford a racemic mixture of diastereomers (81/19 ratio). Chromatography on silica gel (30% EtOAc/hexanes) afforded 3 fractions [total of 148 mg (69%)], 49 mg as the diastereomeric mixture, 33 mg of the minor isomer as a clear oil, and 66 mg of the major isomer as a white solid (mp: 60-61°C).
IR cm-1: Major (KBr): 1677 (CO), Minor (neat): 1682 (CO).
1H-NMR (CDCl3) d: Major: 0.95 (s, 9H, CH3), 2.02 (m, 4H, H-3 and H-4), 3.69 (s, 3H, OCH3), 3.95 (dd, 1H, J=5.8, 9.0 Hz, H-2), 5.77 (d, 1H, J=2.7 Hz, H-6), 6.13 (d, 1H, J=10.0 Hz, H-9), 6.80 (dd, 1H, J=2.7, 10.0 Hz, H-10); Minor: 0.92 (s, 9H, CH3), 2.0 (m, 4H, H-3 and H-4), 3.67 (s, 3H, OCH3), 3.89 (dd, 1H, J=5.9, 8.7 Hz, H-2), 5.67 (d, 1H, J=2.7 Hz, H-6), 6.14 (d, 1H, J=9.9 Hz, H-9), 6.89 (dd, 1H, J=2.7, 9.9 Hz, H-10).
13C-NMR (CDCl3) d: Major: 25.8 (tBu CH3), 27.6 (C-3), 33.6 (tBu C), 38.3 (C-4), 54.9 (OCH3), 79.4 (C-5), 88.9 (C-2), 117.4 (C-6), 126.1 (C-9), 149.5 (C-7), 151.0 (C-10), 181.0 (CO); Minor: 26.1 (tBu CH3), 27.5 (C-3), 33.8 (tBu C), 38.1 (C-4), 55.0 (OCH3), 79.6 (C-5), 88.8 (C-2), 117.4 (C-6), 126.3 (C-9), 149.7 (C-7), 151.0 (C-10), 181.3 (CO).
MS m/e (rel %): Major: 236 [M+] (100), 221 (16), 180 (59), 179 (38), 153 (98), 137 (31), 119 (17); Minor: 236 [M+] (36), 193 (5), 179 (11), 166 (13), 153 (100), 147 (7).
Anal. calc. for C14H20O3: C 71.14, H 8.55; found: C 71.39, H 8.82.
Acknowlegment
The author is thankful for the financial support provided by the University of Northern British Columbia.
Reference
1. Plourde G.L. Tetrahedron Letters 2002, 43, 3597-3599.
© 2003 MDPI. All rights reserved.