| Molbank 2003, M329 |
5-{4-Nitro-3-[1-(toluene-4-sulphonyl)-hexyl]-phenyl}-10,15,20-triphenylporphyrin Zinc(II)
Stanislaw Ostrowskia,b) and Agnieszka Mikusa)
a Institute of Chemistry, University of Podlasie, ul. 3 Maja 54, PL-08-110 Siedlce, Poland
E-mail: [email protected]
b Institute of Organic Chemistry, Polish Academy of Sciences, ul. Kasprzaka 44/52, PL-01-224 Warszawa, Poland
E-mail: [email protected]
Received: 04 April 2003 / Accepted: 05 April 2003 / Published: 03 May 2003
Keywords: Tetraphenylporphyrin derivatives, Carbanions, Sulphones, Alkylation, Zinc
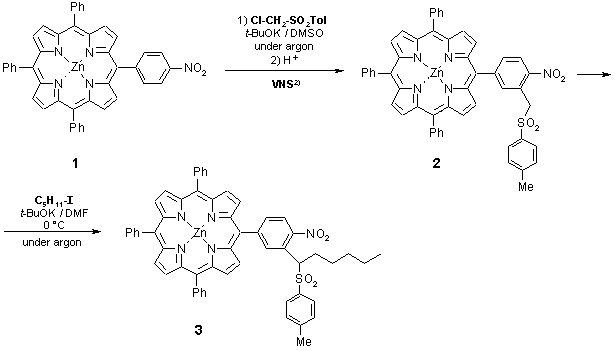
As part of ongoing research programme we synthesised the title meso-tetraarylporphyrin derivative possessing a high degree of complexity in one phenyl ring. This compound is valuable potential intermediate for reactions, leading to biologically active porphyrins, such as PDT agents.1) We used for this purpose the Vicarious Nucleophilic Substitution of Hydrogen reaction (VNS)2) and subsequent alkylation of the intermediate 2 by n-pentyl iodide.

To a stirred solution of t-BuOK (87 mg, 0.78 mmol) in anhydrous DMSO (2.5 ml, under argon) a solution of 5-(4-nitrophenyl)-10,15,20-triphenylporphyrin zinc(II)3) (1; 79 mg, 0.11 mmol) and chloromethyl para-tolyl sulphone (45 mg, 0.22 mmol) in DMSO (1.5 ml) was added dropwise via syringe at room temp. during ca 5 min. After additional 30 min of stirring the mixture was poured into 3% HCl containing ice (30 ml). The precipitate was filtered, washed with water, and then dissolved in CHCl3. After drying with anhydrous Na2SO4 and evaporation of the solvent the 5-[4-nitro-3-(toluene-4-sulphonylmethyl)-phenyl]-10,15,20-triphenylporphyrin zinc(II) (2; described in earlier literature3)) was isolated by column chromatography (silica gel 200-300 mesh, Merck AG; eluent: CHCl3/n-hexane - 3:1); yield - 86 mg, 88%.
In a round-bottom flask (10 ml), the solution of 5-[4-nitro-3-(toluene-4-sulphonylmethyl)-phenyl]-10,15,20-triphenylporphyrin zinc(II) (2; 86 mg, 0.096 mmol) in DMF (2.5 ml) was cooled to 0°C. Then, t-BuOK (45 mg, 0.40 mmol) was added in a one portion and the mixture was stirred for 15 min under argon. To this mixture, 1-iodopentane (79 mg, 0.40 mmol) in DMF (1 ml) was added at 0°C. The reaction was continued at this temp. for ca 3 h until completion (TLC monitoring; CHCl3/n-hexane - 3:1). The mixture was poured onto water with ice (40 ml), the product was extracted with CHCl3 (3 x 10 ml), and the organic layer was washed with H2O (2 x 20 ml). After drying over MgSO4 and evaporation of the solvent, the crude product was purified by column chromatography (silica gel 200-300 mesh, Merck AG) using CH2Cl2/n-hexane mixture (1:1) as eluent. Yield of pure deep-purple solid 5-{4-nitro-3-[1-(toluene-4-sulphonyl)-hexyl]-phenyl}-10,15,20-triphenylporphyrin zinc(II) (3) - 77 mg (83%).
5-[4-Nitro-3-(toluene-4-sulphonylmethyl)-phenyl]-10,15,20-triphenylporphyrin Zinc(II) (2):
M.p. > 300°C.
1H NMR (CDCl3, 200 MHz): 9.02 (d, J = 4.8 Hz, 2 H, Hb-pyrrole), 8.98 (s, 4 H, Hb-pyrrole), 8.83 (d, J = 4.8 Hz, 2 H, Hb-pyrrole), 8.37 (s, 1 H, H-Ar(NO2)), 8.30-8.17 (m, 8 H, 2 H of H-Ar(NO2) and 6 H of H-Ph), 7.84-7.68 (m, 9 H, H-Ph), 7.66 (apparent d, J = 8.5 Hz, 2 H, H-Tol), 7.38 (apparent d, J = 8.5 Hz, 2 H, H-Tol), 5.08 (s, 2 H, CH2), 2.29 (s, 3 H, CH3).
UV-VIS (CH2Cl2), lmax (log e): 590 (3.62), 549 (4.02), 420 (5.19, Soret), 348 nm (3.90).
MS (EI), m/z (% rel. int.): 893 (21), 891 (27), 889 (34) [isotopic M+.], 739 (3), 737 (5), 735 (9), 723 (6), 721 (10), 719 (15), 691 (9), 613 (5), 306 (4), 299 (4), 173 (14), 156 (14), 139 (10), 106 (15), 92 (52), 91 (100), 77 (10), 65 (27), 64 (53).
HR-MS (EI) Calcd for C52H35N5O4SZn: 889.1701; Found: 889.1699.
5-{4-Nitro-3-[1-(toluene-4-sulphonyl)-hexyl]-phenyl}-10,15,20-triphenylporphyrin Zinc(II) (3):
M.p. > 300°C.
IR (neat, cm-1): 3103, 3055 & 3021 (CHaromat.); 2956, 2921 & 2850 (CH3, CH2); 1597; 1524 & 1340 (NO2); 1320 & 1147 (SO2).
1H NMR (CDCl3, 200 MHz): 9.11 (d, J = 4.8 Hz, 1 H, Hb-pyrrole), 9.02-8.95 (m, 3 lines, 6 H, Hb-pyrrole), 8.77 (d, J = 4.6 Hz, 1 H, Hb-pyrrole), 8.66 (d, J = 1.7 Hz, 1 H, H-Ar(NO2)), 8.30 (part of AB coupled with another proton, J = 8.3, 1.7 Hz, 1 H, H-Ar(NO2)), 8.28-8.19 (m, 6 H, H-Ph), 8.18 (part of AB, J = 8.3 Hz, 1 H, H-Ar(NO2)), 7.83-7.73 (m, 9 H, H-Ph), 7.70 (apparent d, J = 8.2 Hz, 2 H, H-Tol), 7.33 (apparent d, J = 8.2 Hz, 2 H, H-Tol), 5.69 (dd, J = 10.8, 4.2 Hz, 1 H, CH(SO2Tol)), 2.50-2.38 (m, 2 H, CH2), 2.41 (s, 3 H, CH3-Tol), 1.37-1.22 (m, 6 H, 3xCH2), 0.85 (t, J = 6.9 Hz, 3 H, CH3).
UV-VIS (CHCl3), lmax (log e): 596.5 (3.56), 556 (4.09), 518 (3.38), 423.5 (5.39, Soret), 356 (3.90), 333 nm (4.10).
LSIMS(+), m/z (% rel. int.): 961 (2), 960 (3, M+H), 959 (3), 958 (3), 803 (2), 601 (2), 523 (3), 395 (7), 369 (3), 307 (12), 154 (100).
Elemental analysis calcd. for C57H45N5SO4Zn (961.46): C - 71.21, H - 4.72, N - 7.28. Found: C - 70.75, H - 5.26, N - 6.32.
References
1. Hsi,
R.A.; Rosenthal, D.I.; Glatstein, E.
Drugs 1999, 57, 725.
2. a) Makosza, M.; Wojciechowski, K. Liebigs Ann. / Recueil 1997, 1805.
b) Ostrowski, S.;
Shim, Y.K. Bull. Korean Chem. Soc. 2001, 22, 9.
3. Ostrowski, S.; Mikus, A.; Shim, Y.K.; Lee, J.-Ch.; Seo, E.-Y.; Lee, K.-I.;
Olejnik, M.
Heterocycles 2002, 57, 1615.
© 2003 MDPI. All rights reserved.