| Molbank 2003, M330 |
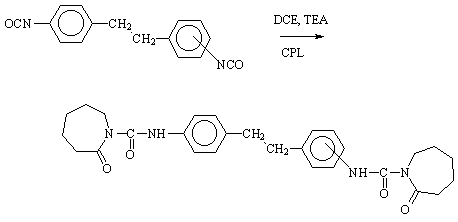
1,2-Bis(2-oxo-azepane-1-carboxylic acid-p-phenyl-amide)ethane and 1-(2-Oxo-azepane-1-carboxylic Acid-o-phenyl-amide)-2-(2-oxo-azepane-1-carboxylic acid-p-phenyl-amide)ethane
Elena Scortanu* and Adrian A. Caraculacu
Romanian Academy, Institute of Macromolecular Chemistry "Petru Poni", Aleea Grigore Ghica Voda, Nr. 41 A, Iasi 6600, Romania
Phone: 0232-260332, Fax: 0232-211299, E-mail: [email protected]
Received: 22 February 2003 / Accepted 4 June 2003 / Published 8 June 2003

The experimental procedure follows the general synthesis by the addition reaction of isocyanates with e-caprolactam (CPL) [1, 2]. A solution of 1,2-bis(p-isocyanato-phenyl)ethane (4,4'-dibenzyl diisocyanate) [3, 4] (132 g, 0.5 mol) and 2-oxo-azepane (e-caprolactam) (113 g, 1.0 mol) in 500 mL 1,2-dichloroethane (DCE) was maintained at 60°C for 0.5 h. The homogeneous mixture was treated with 2 g triethyl amine (TEA) (used as a catalyst) and stirred at 70-80°C for another 3 h. The progress of reaction was followed by IR spectroscopy. At the end of reaction period, the mixture was cooled while stirring. When the crystalline product was obtained, it was isolated by filtration, washed with acetone or methanol and dried at 100°C. The product was a white crystalline solid (223 g, 91% yield). The adduct of 1-(o-isocyanato-phenyl)-2-(p-isocyanato-phenyl)ethane (2,4'-dibenzyl diisocyanate) with e-caprolactam was obtained by the same method.
1,2-Bis(2-oxo-azepane-1-carboxylic acid-p-phenyl-amide)ethane
Thermal behavior: M.p.> 194°C with decomposition (thermo-optical method).
IR (KBr, cm-1): 3200, 3150 mw (N-H), 3100 mw, 3040 mw (C-H), 2970-2940 ms (CH2), 2860 mw (CH2), 1720-1690 s (CO-N<), 1650 ms (CONH), 1590 s (CONH, p-Ar), 1540 s (CONH), 1510 ms (p-Ar), 1440 m, 1410 ms, 1390 s (CH2, p-Ar), 1345 mw, 1330 m, 1320 m, 1280 mw (CH2, C-O), 1230 m, 1210 m (CH2, C-N, C-O), 1170 s, 1150 ms, 1140 m (CH2), 970 ms (C-C), 830 ms (p-Ar), 760 m (C-N).
1H NMR (60 MHz, d, DMSO-d6, 90°C): 7.6 (s, NH, 2H), 7.3, 7.18, 7.05, 6.9 (m, Ar, 8H), 4.08, 3.95 (m, CH2-N<, 4H), 2.84, 2.7 (d, CH2-Ar, 4H), 2.3, 2.1, 1.74 (m, CH2, 16H) ppm.
Elemental analysis: Calculated for: C28H34N4O4: C%= 68.55; H%= 6.99; N%= 11.42. Found: C%= 68.92; H%= 7.07; N%= 11.68.
1-(2-oxo-azepane-1-carboxylic acid-o-phenyl-amide)-2-(2-oxo-azepane-1-carboxylic acid-p-phenyl-amide)ethane
Thermal behavior: The melting process started at 140°C; over 154°C a mesophase (birefringence) was observed up to 164°C (the isotropization temperature-Ti), followed by the decomposition process (thermo-optical method).
IR (KBr, cm-1): 3200-3120 mw (N-H), 3050 mw (C-H), 2920 m (CH2), 2840 mw (CH2), 1720-1690 s (CO-N<), 1650 ms (CONH), 1590 s (CONH, o,p-Ar), 1540-1520 s (CONH, o,p-Ar), 1440 ms, 1400 s (CH2, o,p-Ar), 1350 m, 1330 m, 1280 mw (CH2, C-O), 1260 mw, 1230 m, 1210 ms (CH2, C-N, C-O), 1170 s, 1150 s (CH2), 1080 m (p-Ar), 970 ms, 950 m (C-C), 900 m (C-C, C-N), 840 mw, 810 m (p-Ar), 760 s ( o-Ar, C-N).
1H NMR (60 MHz, d, DMSO-d6, 90°C): 7.75, 7.62 (m, NH, 2H), 7.3, 7.18, 7.08, 6.95, 6.82 (m, Ar, 8H), 3.95 (m, CH2-N<, 4H), 2.88 (s, CH2-Ar, 4H), 2.73 (s, CH2-CO, 4H), 2.1, 1.8 (s, CH2, 12H) ppm.
Elemental analysis: Calculated for: C28H34N4O4: C%= 68.55; H%= 6.99; N%= 11.42. Found: C%= 68.44; H%= 7.31; N%= 11.26.
References:
1. Savostianoff, E. Inf. Chem. 1981, 119-126, 216-217, 1312.
2. Kovalenko, L. G.; et al. Plast. Massy 1986, 11, 34.
3. Caraculacu, A. A.; et al., Rom. Pat. 81,947 1983.
4. Caraculacu, A. A.; Caraculacu, G. J. Macromol. Sci. Chem. 1985, A22, 631.
© 2003 MDPI. All rights reserved.