| Molbank 2003, M331 |
1,2-Bis(p-phenyl-thiocarbamic Acid-S-benzothiazol-2-yl-ester)ethane and 1-(o-Phenyl-thiocarbamic Acid-S-benzothiazol-2-yl-ester)-2-(p-phenyl-thiocarbamic Acid-S-benzothiazol-2-yl-ester)ethane
Elena Scortanu* and Adrian A. Caraculacu
Romanian Academy, Institute of Macromolecular Chemistry "Petru Poni", Aleea Grigore Ghica Voda, Nr. 41 A, Iasi 6600, Romania
Phone: 0232-260332, Fax: 0232-211299, E-mail: [email protected]
Received: 22 February 2003 / Accepted 4 June 2003 / Published 8 June 2003

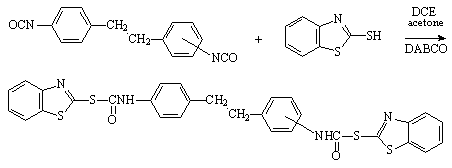
The experimental procedure follows the general synthesis of blocked isocyanates [1, 2]. A solution of mercaptobenzothiazole (MBT) was obtained by mixing 23 g (0.1373 mol) with 250 mL 1,2-dichloroethane (DCE) and 50 mL acetone, then heating gradually to 35-50°C for 0.5 h until the mixture became homogeneous. A solution of 1,2-bis(p-isocyanato-phenyl)ethane (4,4'-dibenzyl diisocyanate) [3, 4] (17 g, 0.0644 mol) in 100 mL DCE was added to this mixture under stirring during 0.25 h and then 0.2 g diazabicyclo[2.2.2]octane (DABCO) also was added. The mixture was stirred at 68-72°C for 6 - 8 h. After cooling, the light yellow solid was isolated by filtration, washed with DCE and acetone (to remove the excess of MBT) and dried at 90-100°C (22.5 g, 57.73% yield).
The synthesis of the nonsymmetrical isomer is described as follows. To a solution of MBT (5 g, 0.029895 mol) in 100 mL DCE and 15 mL acetone, 1-(o-isocyanato-phenyl)-2-(p-isocyanato-phenyl)ethane (2,4'-dibenzyl diisocyanate) (3.42 mL, 3.8 g, 0.01439 mol) and 0.1 g DABCO was added. The mixture was stirred for 10 h at 70-72°C. After cooling, the solid product was isolated by filtration, washed several times with acetone and dried (3 g, 34.84% yield).
1,2-Bis(p-phenyl-thiocarbamic acid-S-benzothiazol-2-yl-ester)ethane
Thermal behavior: M.p. >328°C (decomposition), (thermo-optical method)
IR (KBr, cm-1): 3320 m (NH), 3030 mw (C-H), 2945 mw (CH2), 2850 mw (CH2), 1720-1700 m (NHCOS), 1670-1650 m (CONH), 1600 ms (p-Ar), 1550 s, 1530 s, 1510 s (SCONH), 1500 m (sh, p-Ar), 1410 m (C-S, MBT), 1320 m (MBT, p-Ar), 1240 ms (C-S, C-O), 1200 m (sh, C-N, C-O), 1080 m (C-O, C-N), 835 m (p-Ar), 750 mw (C-N, MBT), 660 mw (C-S).
1H NMR (60 MHz, d, DMSO-d6, 80°C): 8.95, 8.2 (NH, 2H), 7.2, 7.08, 6.95, 6.8 (m, Ar, 16H), 3.62 (s, CH2-Ar, 4H) ppm.
Elemental analysis: Calculated for: C30H22N4O2S4: C%= 60.18; H%= 3.37; N%= 9.36; S%= 21.42. Found: C%= 60.58; H%= 3.73; N%= 9.87.
1-(o-phenyl-thiocarbamic acid-S-benzothiazol-2-yl-ester)-2-(p-phenyl-thiocarbamic acid-S-benzothiazol-2-yl-ester)ethane
Thermal behavior: M.p.> 228°C (decomposition), (thermo-optical method).
IR (KBr, cm-1): 3300 ms (NH), 3040 w (C-H), 2940 w (CH2), 2865 w (CH2), 1700 m (sh, NHCOS), 1680-1655 ms (CONH), 1610-1590 ms (Ar), 1550 s, 1515 s (SCONH), 1500 ms (sh, Ar), 1450 ms, 1410 m (C-S, MBT), 1330-1300 m (MBT), 1260-1240 ms (C-S, C-O), 1180 m (sh, C-N, C-O), 1090 mw (C-O), 830 mw (p-Ar), 760 m (o-Ar, NNCOS), 700 w, 670 w (C-S).
1H NMR (60 MHz, d, DMSO-d6, 60°C): 8.56, 8.2, 7.95 (t, NH, 2H), 7.65, 7.45, 7.3, 7.2, 7.07, 6.95, 6.85 (m, Ar, 16H), 3.56 (m, CH2-Ar, 4H) ppm.
Elemental analysis: Calculated for: C30H22N4O2S4: C%= 60.18; H%= 3.37; N%= 9.36; S%= 21.42. Found: C%= 60.47; H%= 3.85; N%= 9.65.
References:
1. Savostianoff, E. Inf. Chem. 1981, 119-126, 216-217, 1312.
2. Kovalenko, L. G.; et al. Plast. Massy 1986, 11, 34.
3. Caraculacu, A. A.; et al., Rom. Pat. 81,947 1983.
4. Caraculacu, A. A.; Caraculacu, G. J. Macromol. Sci. Chem. 1985, A22, 631.
© 2003 MDPI. All rights reserved.