| Molbank 2003, M333 |
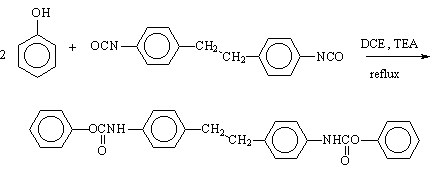
1,2-Bis(p-phenyl-carbamic Acid Phenyl Ester)ethane
Elena Scortanu* and Adrian A. Caraculacu
Romanian Academy, Institute of Macromolecular Chemistry "Petru Poni", Aleea Grigore Ghica Voda, Nr. 41 A, Iasi 6600, Romania
Phone: 0232-260332, Fax: 0232-211299, E-mail: [email protected]
Received: 22 February 2003 / Accepted 4 June 2003 / Published 8 June 2003

The experimental procedure follows the general synthesis by the addition reaction of isocyanates with phenols [1, 2]. Thus, a solution of 1,2-bis(p-isocyanato-phenyl)ethane (4,4'-dibenzyl diisocyanate) [3, 4] (50 g, 0.189 mol), phenol (37 g, 0.394 mol), in 350 mL 1,2-dichloroethane (DCE) was maintained under stirring at 40-50°C for 0.5 h. An endothermic reaction began when 0.5 mL triethylamine (TEA) was added to the solution. The reaction mixture was maintained at the reflux temperature for 2-4 h under stirring and the product precipitated as the reaction progressed. The progress of the reaction was followed by IR spectroscopy. After cooling, the solid was isolated by filtration, washed with acetone or methanol and dried at 100°C. The product was a white crystalline solid (83.5 g, 97.55% yield).
Thermal behavior: M.p.: 228-245°C (decomposition), (thermo-optical method).
IR (KBr, cm-1): 3300 m (N-H), 3050 mw (C-H), 2900 mw, 2860 w (CH2), 1720-1690 s (CO-NH), 1600-1580 m (p-Ar), 1540 s (CONH, Ar), 1480 m (p-Ar), 1420 m (p-Ar), 1320 m (C-O), 1220 s (C-N, C-O), 830 m (p-Ar), 730 mw, 700 mw (C-N, Ar).
1H NMR (60 MHz, d, DMSO-d6, 70°C): 8.88, 8.18 (NH, 2H), 7.31-6.9, 6.8-6.3 (m, Ar, 18H), 2.85, 2.75 (d, CH2-Ar, 4H) ppm.
Elemental analysis: Calculated for: C28H24N2O4: C%= 74.32; H%= 5.35; N%= 6.19. Found: C%= 74.09; H%= 5.55; N%= 6.65.
References:
1. Savostianoff, E. Inf. Chem. 1981, 119-126, 216-217, 1312.
2. Kovalenko, L. G.; et al. Plast. Massy 1986, 11, 34.
3. Caraculacu, A. A.; et al., Rom. Pat. 81,947 1983.
4. Caraculacu, A. A.; Caraculacu, G. J. Macromol. Sci. Chem. 1985, A22, 631.
© 2003 MDPI. All rights reserved.