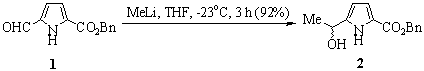
| Molbank 2003, M337 |
(R,S)-Benzyl 5-(1-Hydroxyethyl)-1H-pyrrole-2-carboxylate
Derek C. Martyn and Andrew D. Abell*
Department of Chemistry, The University of Canterbury, Private Bag 4800, Christchurch, New Zealand
Fax: (+64 3) 3642110, E-mail: [email protected]
Received: 22 February 2003 / Accepted 22 May 2003 / Published 1 June 2003
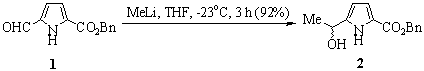
A stirred solution of the pyrrole benzyl ester 1 [1] (1.20 g, 5.23 mmol, 1 equiv) in dry tetrahydrofuran (100 mL) under an inert atmosphere was cooled to -23°C (dry ice/carbon tetrachloride). Methyllithium (3.27 mL of a 1.6 M solution in ether, 5.23 mmol, 1 equiv) was added over 10 min, and the solution was stirred at -23°C for 1.5 h. 1H NMR analysis of an aliquot from the reaction indicated a 1.2:1 mixture of starting material to product. A further 3.93 mL of methyllithium (6.28 mmol, 1.2 equiv) was added over 10 min, and the solution was stirred at -23°C for an additional 1.5 h. The resultant solution was poured onto an ether/ice bath, and upon melting of the ice the layers were separated. The organic layer was washed with water (2 x 50 mL), aqueous saturated brine (50 mL), dried (MgSO4), and the solvent was removed by evaporation under reduced pressure. Flash chromatography on silica (ethyl acetate/petroleum ether, 3:1) afforded the title compound 2 (1.18 mg, 92%) as a light pink solid.
mp 82-84ºC.
IR (CHCl3) 1676.0, 2359.7, 2971.1, 3165.5, 3389.2.
1H NMR (CDCl3, 500 MHz) d 1.54 (d, 3H, J = 6.8 Hz, CH(OH)CH3), 2.55 (s(br), 1H, CHOH), 4.94 (q, 1H, J = 6.2 Hz, CHOH), 5.29 (s, 2H, CH2Ph), 6.05 (m, 1H, pyrrole H4), 6.89 (m, 1H, pyrrole H3), 7.31-7.41 (m, 5H, ArH), 9.67 (s(br), 1H pyrrole NH).
13C NMR (CDCl3, 75 MHz) d 23.1 (CH(OH)CH3), 63.9 (CHOH), 65.9 (CH2Ph), 106.4 (pyrrole C4), 116.4 (pyrrole C3), 121.3 (pyrrole C2), 127.9, 128.1, 128.5, 136.0 (ArC), 141.8 (pyrrole C5), 161.6 (CO2).
HRMS (M+) Calcd for C14H15NO3: 245.1052. Found: 245.1055.
Reference
1. Noss, L.; Liddell, P. A.; Moore, A. L.; Moore, T. A.; Gust, D. J. Phys. Chem. B 1997, 101, 458.
Sample availability: available from the authors.
© 2003 MDPI. All rights reserved.