| Molbank 2003, M341 |
Methyl 2-Amino-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate
Abdullah Mohamed Asiri
Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, P.O. Box 80203, Saudi Arabia.
Tel.(+966)-2-6952293, Fax (+966)-2-6952293, E-mail: [email protected]
Received: 5 March 2003 / Accepted 4 June 2003 / Published 21 June 2003
Keywords: thiophene, Gewald reaction, cyclopentanone
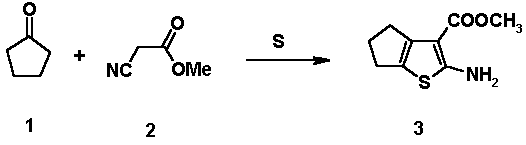
To a mixture contaning cyclopentanone (8.49 g, 0.1 mol), methyl cyanoacetate (10.0 g, 0.1 mol) and sulfur (3.21 g, 0.1 mol) in methanol (20 ml), morpholine (10 ml) was added dropwise over a period of 30 minutes at 35 °C. The reaction mixture was heated at 45 °C with stirring for 3 hrs, then was allowed to cool to room temperature. The precipated powder was filtered and washed with ethanol (2x30 ml). Recrystallization from ethanol gave pale yellow powder (13.0g, 65 %).
M.p. 185-186°C (Ethanol, uncorrected).
IR (KBr) (cm-1; KBr Disk) 3410, 3293 (NH), 1652 (C=O), 1596 (NH), 1351 (C-N).
1H-NMR (400 MHz; CDCl3; Me4Si, dH): 2.31 (2H, m, CH2CH2CH2), 2.71 ( 2H, t, J = 6.70 Hz, CH2CH2CH2), 2.2.8 (2H, t, J = 7.00 Hz, CH2CH2CH2), 3.78 (3H, s, CH3O), 5.87 ( 2H, bs, NH2).
13C-NMR (dC): 26.12 (CH2CH2CH2), 27.82 (CH2CH2CH2), 29.66 (CH2CH2CH2), 49.56 (CH3O), 99.66, 119.16, 141.01, 164.98, 166.99 (CO).
Elemental Analysis: Calculated for C9H13NO2S (199.27): C 54.25 %, H 6.58 %, N 7.03 %, S 16.09 %; found : C 54.11 %, H 6.46 %, N 7.03 %, S 15.89 %.
References
1. Gewald, K.; Schinke, E.; Böttcher, H. Chem. Ber. 1966, 99, 94-100.
2. Gewald, K.; Neumann, G. Chem. Ber. 1968, 101, 1933-1939.
Sample availability: available from the author.
© 2003 MDPI. All rights reserved.