| Molbank 2003, M345 |
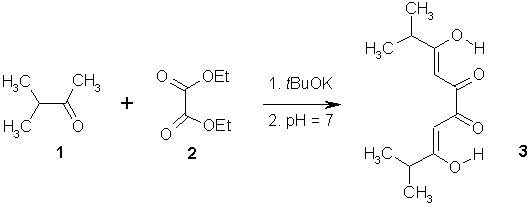
3,8-Dihydroxy-2,9-dimethyl Deca-3,7-diene-5,6-dione
Ibrahim Bouabdallah, Ismail Zidane, Fouad Malek, Rachid Touzani, Mohamed El Kodadi and Abdelkrim Ramdani*
Laboratoire de Chimie Organique Physique, Département de Chimie, Faculté des Sciences, Université Mohamed Premier, BP 524, 60000, Oujda, Maroc
Email: [email protected]
Received: 11 March 2003 / Accepted: 24 June 2003 / Published 28 June 2003

The product 3 was prepared according to a reported procedure [1,2]. A suspension of potassium tert-butoxide (2.93 g, 0.02 mol) was stirred in dry diethyl ether (45 mL) at 0°C. A mixture of 3-methyl-2-butanone 1 (1.72 g, 0.02 mol) and diethyloxalate (1.46 g, 0.01 mol) was added dropwise over 10 min. and the mixture was stirred for one day (25°C). The salt was precipitated and collected by filtration, dissolved in water (50 mL) and neutralized with glacial acetic acid to gives the product 3 as a yellow solid (1.21 g, 54%).
Melting point: 33-35°C.
IR. (KBr, cm-1): 2980(CH3), 1560(C=O), 1080(C-O), 820(C-H).
HRMS (C12H18O4): calc'd 226.1205; found 226.1217.
1H-NMR (200 MHz, CDCl3, d, ppm): 6.43 (s, 2H, =C-H); 2.72 (m, 2H, C-H); 1.24 (d, 12H, CH3, J=5.2 Hz).
13C-NMR (50.4 MHz, CDCl3, d, ppm): 208.1; 171.5; 97.8; 39.7; 19.1.
References and Notes
1. Touzani, R.; Benhadda, T.; Elkadiri, S.; Ramdani, A.; Maury, O.; Lebozec, H.; Toupet, L.; Dixneuf., P. H. New. J. Chem. 2001, 25, 391.
2. Finar., I. L. J. Chem. Soc. 1958, 4094.
Sample availability: available from the authors.
© 2003 MDPI. All rights reserved.