| Molbank 2002, M348 |
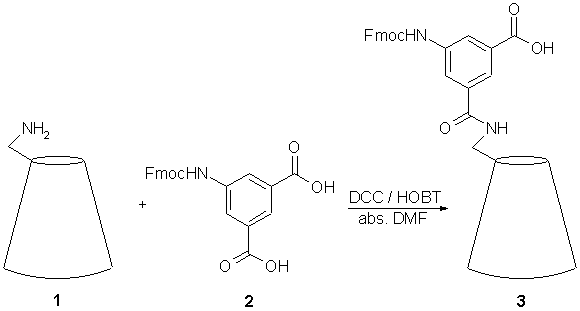
6-[5-(9H-Fluoren-9-ylmethoxycarbonylamino)-isophthalate]-amido-6-deoxy-b-cyclodextrine
Thorsten Graf and Burkhard Koenig*
Department of Organic Chemistry, Faculty of Science IV, University of Regensburg, Universitätsstrasse 31, 93051 Regensburg, Germany
Fax (+49) 941 943 171; E-mail: [email protected]
Received: 31 March 2003 / Accepted: 24 June 2003 / Published: 28 June 2003

6-Amino-6-deoxy-b-cyclodextrine (1) was prepared according to the literature [1,2] in a three step synthesis. The Fmoc protected 5-aminoisophthalic acid is prepared according to the following procedure. To a solution of 15 ml of dioxane and 40 ml of water containing 5-aminoisophthalic acid (500 mg, 2.76 mmol) and Na2CO3 (878 mg, 8.28mmol) a solution of FmocCl (785 mg, 3.04 mmol) in 10 ml of dioxane was added dropwise at 0° within 15 min. The mixture was stirred for 20 h at room temp., 2 M HCl solution was added until a pH of 2 is reached. The crude product was extracted with 250 ml of ethyl acetate. The organic phase was dried over Na2SO4 and evaporated under reduced pressure. The residual was then stirred in 100 ml of methylene chloride for 2 h, filtered, washed with methylene chloride and dried under reduced pressure to yield 1.01 g (91%) of crude 2 as a light brown powder.
To obtain the titled product 3 a solution of DCC (66 mg, 0.32 mmol) and HOBT (43 mg, 0.32 mmol) in 20 ml of abs. DMF was added dropwise to a solution of 2 (118 mg, 0.29 mmol) in 20 ml of abs. DMF under nitrogen atmosphere at 0°C within 15 min. To this mixture a solution of 1 (300 mg, 0.26 mmol) in 40 ml of abs. DMF was added dropwise within 15 min. After the mixture was stirred at room temp. for 18 h the solvent was removed at 40°C under reduced pressure. The resulting residual was stirred in 75 ml of acetone for 2 h. The crude product was filtered, washed with acetone and dried under reduced pressure to yield 364 mg (92%) of the title compound 3 as a light brown powder. The crude product was purified by preparative HPLC on a reverse phase column (column: Phenomenex; Luna 10 C18; solvent gradient: water /acetonitril from 0% CH3CN to 95%; flow rate: 10.5 ml/min; retention time of product: 9.3 min; detection: UV absorption 214 and 195 nm).
MP: thermal decomposition above 225°C.
MS (-p ESI, DMSO/MeOH + 10 mmol/l NH4OAc): 647.4 (75%) [M-H-Fmoc]2-, 1295.9 (15%) [M-Fmoc]-, 1518.2 (100%) [M-H]-.
UV/Vis (MeOH/H2O 1:1) lmax [nm] (lg e): 254.6 (5.353), 289.0 (4.777), 300.0 (4.847).
1H-NMR (600 MHz): 3.25 - 3.86 (m, 42H, 7xCD-H2, 7xCD-H3, 7xCD-H4, 7xCD-H5, 7xCD-H6, 7xCD-H6'), 4.24 - 4.35 (m, 2H, 1xCD-6OH, H9), 4.42 - 4.51 (m, 7H, 5xCD-6OH, H8, H8'), 4.79 - 4.86 (m, 6H, 6xCD-H1), 4.96 (d, 3J = 3.4 Hz, 1H, 1xCD-H1), 5.65 - 5.82 (m, 14H, 7xCD-2OH, 7xCD-3OH), 7.36 (dt, 3J = 7.4 Hz, 4J = 0.9 Hz, 2H, H11, H11'), 7.43 (t, 3J = 7.4 Hz, 2H, H12, H12'), 7.77 (d, 3J = 7.4 Hz, 2H, H10, H10'), 7.91 (d, 3J = 7.4 Hz, 2H, H13, H13'), 8.03 (s, 1H, H2), 8.08 (s, 1H, H4,), 8.30 (s, 1H, H6), 8.34 (s, 1H, CD-NH), 10.01 (s, 1H, NH), 13.10 (br, 2H, COOH).
13C-NMR (150 MHz): 46.5 (+, C9), 59.4 (-, CD-C6), 59.5 (-, CD-C6), 59.7 (-, CD-C6), 59.9 (-, CD-C6), 65.7 (-, C8), 69.5 (+, CD-CH), 71.9 (+, CD-CH), 71.9 (+, CD-CH), 72.0 (+, CD-CH), 72.1 (+, CD-CH), 72.3 (+, CD-CH), 72.4 (+, CD-CH), 72.9 (+, CD-CH), 81.2 (+, CD-CH), 81.2 (+, CD-CH), 81.3 (+, CD-CH), 81.4 (+, CD-CH), 81.6 (+, CD-CH), 81.7 (+, CD-CH), 83.8 (+, CD-CH), 101.6 (+, CD-C1), 101.7 (+, CD-C1), 101.8 (+, CD-C1), 101.9 (+, CD-C1), 102.1 (+, CD-C1), 120.1 (+, C13, C13'), 121.1 (+, C6), 121.5 (+, C4), 121.9 (+, C2), 125.1 (+, C10, C10'), 127.0 (+, C11, C11'), 127.6 (+, C12, C12'), 135.5 (Cquat, C1), 139.1 (Cquat, C3), 140.7 (Cquat, C14, C14'), 143.6 (Cquat, C15, C15'), 153.3 (Cquat, C7), 165.9 (Cquat, C17), 166.8 (Cquat, C16).
Acknowledgment: We thank the Wacker-Chemie GmbH Burghausen, Germany, for their support.
References
1. Matsui, Y.; Okimoto, A. Bull. Chem. Soc. Jpn. 1978, 51, 3030 - 3034.
2. Hamasaki, K; Ikeda, H.; Nakamura, A.; Ueno, A; Toda, F.; Suzuki, I; Osa, T. J. Am. Chem. Soc. 1993, 115, 5035 - 5040.
© 2002 MDPI. All rights reserved.