| Molbank 2003, M349 |
1-(o-Hydroxyphenyl)-3-phenylpropenone N-Benzoylhydrazone
Antigoni Kotali* and Ioannis S. Lafazanis
Laboratory of Organic Chemistry, Department of Chemical Engineering, College of Engineering, University of Thessaloniki, Thessaloniki 54006, Greece
E-mail: [email protected]
Received: 13 March 2003 / Accepted: 26 June 2003 / Published 28 June 2003
Keywords: o-Hydroxyaryl ketones, carbonylhydrazones, 1-(o-hydroxyphenyl)-3-phenylpropenone.
As part of a research programme targeting novel molecules derived from o-hydroxyaryl ketone hydrazones[1], we synthesised 1-(o-hydroxyphenyl)-3-phenylpropenone N-benzoylhydrazone.
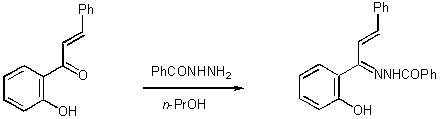
1-(o-Hydroxyphenyl)-3-phenylpropenone was prepared according to the literature method [2]. Benzoic hydrazide is commercially available and was supplied by Aldrich. Benzoic hydrazide (0.63 g, 4.5 mmol) was added to a solution of 1-(o-hydroxyphenyl)-3-phenylpropenone (1 g, 4.5 mmol) in propanol-1 (10 mL). The reaction mixture was refluxed for 24 hours. It was then allowed to cool at room temperature. Subsequently, it was stored in the refrigerator overnight. Filtration of the precipitate, which was formed, afforded (1.38 g, 90 %) of the desired 1-(o-hydroxyphenyl)-3-phenylpropenone N-benzoylhydrazone as white crystals. The product was identified by 1H NMR, 13C NMR and MS and it was subjected to elemental analysis without further purification.
M.p. 201-202 °C.
1H NMR (400 MHz, DMSO-d6): 6.94-7.03 (m, 3H), 7.33-7.59 (m, 10H), 7.8-8.15 (m, 3H), 10.18 (s, 1H), 10.91 (s, 1H).
13C NMR (100 MHz, DMSO-d6): 116.9, 118.1, 120.0, 120.6, 121.9, 125.3, 126.1, 127.0, 127.8, 128.4, 128.7, 128.9, 129.2, 131.3, 132.0, 132.4, 140.2, 157.2.
MS m/z (ES+): 707 [2M+Na]+, 365 [M + Na]+.
Anal. Calc. for C22H18N2O2: C 77.17, H 5.29, N 8.18; found: C 77.20, H 5.27, N, 8.19.
References and Notes
1. Kotali, A.; Harris, P. A. Org. Prep. Proc. Int. 1994, 26, 159 - 192.
2. Thakkar, K.; Cushman, K. Tetrahedron Lett. 1994, 35, 6441 - 6444.
Sample availability: available from the authors and MDPI.
© 2003 MDPI. All rights reserved.