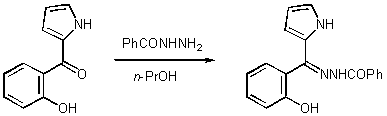
| Molbank 2003, M351 |
2-(2´-Hydroxybenzoyl)pyrrole N-Benzoylhydrazone
Antigoni Kotali
Laboratory of Organic Chemistry, Department of Chemical Engineering, College of Engineering, University of Thessaloniki, Thessaloniki 54006, Greece
E-mail: [email protected]
Received: 13 March 2003 / Accepted: 26 June 2003 / Published 28 June 2003
Keywords: o-Hydroxyaryl ketones, carbonylhydrazones, 2-(2´-hydroxybenzoyl)pyrrole.
In continuation of our interest in using
o-hydroxyaryl ketone hydrazones as starting materials in organic synthesis [1], we synthesised 2-(2´-hydroxybenzoyl)pyrrole
N-benzoylhydrazone.
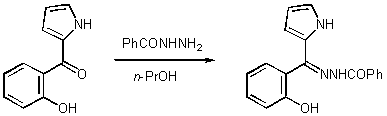
Benzoic hydrazide is commercially available and it was supplied by Aldrich, whereas 2-(2´-hydroxybenzoyl)pyrrole was prepared in three steps according to the literature method [2]. Benzoic hydrazide (0.68 g, 5 mmol) was added to a solution of 2-(2´-hydroxybenzoyl)pyrrole (1 g, 5 mmol) in propanol-1 (10 mL). The reaction mixture was refluxed for 24 hours. It was then allowed to cool at room temperature. Subsequently, it was stored in the refrigerator overnight. Filtration of the precipitate, which was formed, afforded (1.41 g, 92 %) of the desired 2-(2´-hydroxy benzoyl)pyrrole N-benzoylhydrazone as light yellow crystals. The product was identified by 1H NMR, 13C NMR and MS and it was subjected to elemental analysis without further purification.
M.p. 213-215 °C.
1H NMR (300 MHz, DMSO-d6): 6.93-7.19 (m, 2H), 7.33-7.61 (m, 7H), 7.62-7.79 (m, 3H), 9.73 (s, 1H), 10.79 (s, 1H), 12.03 (s, 1H).
13C NMR (75 MHz, DMSO-d6): 114.0, 111.8, 117.4, 119.2, 120.2, 123.5, 124.0, 127.1, 127.4, 128.5, 130.6, 131.5, 133.6, 133.7, 158.7, 166.4.
MS m/z (ES+): 328 [M+Na]+, 306 [M+1]+.
Anal. Calc. for C18H15N3O2: C 70.81, H 4.95, N 13.76; found: C 70.84, H 5.10, N, 13.81.
References and Notes
1. Kotali, A.; Harris, P. A. Org. Prep. Proc. Int. 1994, 26, 159 - 192.
2. Petruso, S.; Bonanno, S.; Caronna, S.; Ciofalo, M.; Maggio, B.; Schillaci, D. J. Heterocycl. Chem. 1994, 31, 941 - 945.
Sample availability: available from the authors and MDPI.
© 2003 MDPI. All rights reserved.