| Molbank 2004, M364 |
A. A. jarrahpour*1, M. Motamedifar2, H. Mousavipour1, N. Hadi2 and M. Zarei1
1 Department of Chemistry, College of Sciences, Shiraz University, Shiraz 71454, Iran Tel. 0098 711 2284822, Fax: 0098 711 2280926, E-mail: [email protected], [email protected]
2 Department of Bacteriology & Virology, Shiraz Medical School, Shiraz University Medical Science, Shiraz 71345, Iran
¡¡
Received: 24 October 2003 / Accepted: 11 February 2004 / Published: 24 February 2004
¡¡
Keywords : Schiff base, 3-amino-2-chloropyridine, p-nitrobenzaldehyde, biological activity, ab initio
Schiff bases are reported to show a variety of biological activities such as antibacterial [1-3], antifungal [4-5], anticancer [6-7] and herbicidal [8] activities . Pyridinium compounds have biological activities [9] such as antifungal [10] and antibacterial [10-11] activities. The presence of a chloro moiety in different types of compounds causes them to exhibit pesticidal activity8. Based on these facts , we synthesized 2-chloro pyridin-3-yl¨C(4-nitrobenzylidene) amine 3. According to ab initio calculations at the HF/6-31G (d,p) level, Schiff base 3 at dihedral angel of 42.9 o is about 15.70 kj/mol more stable than the coplanar pyridine and nitrophenyl rings (Figure 1). The titled compound was tested against S. aureus, B. subtilis, K. pneumonia and P. aeruginosa by the disk diffiusion method and was not active at the level of 200 ¦Ìg/disk.

A mixture of 3-amino-2-chloropyridine 1(1.28 g, 10.00 mmol), 4-nitrobenzaldehyde 2 (1.51 g, 10.00 mmol) and anhydrous magnesium sulfate (4.00 g) in dry dichloromethane (40.00 mL) was stirred at room temperature for four hours. The suspension was filtered and washed with dichloromethane.The solvent was evaporated under reduced pressure and Schiff base 3 was formed as a yellow solid which was recrystalized from warm ethanol (2.23 g , 85% ).
m.p.146-148 oC.
IR (KBr) (cm-1): 1340.40 and 1517.90 (NO2), 1600.0 (N=CH), 1633.60 (C=N pyridine ring) .
1H-NMR
(CDCl3) (250 MHz)![]() (ppm): 7.23-8.38 (m , 7 H, Ar-H), 8.54 (s, 1H, CH=N).
(ppm): 7.23-8.38 (m , 7 H, Ar-H), 8.54 (s, 1H, CH=N).
13C- NMR
(CDCl3) (62.90 MHz)![]() (ppm): 123.65-147.18 (2 ph), 161.41 (CH=N).
(ppm): 123.65-147.18 (2 ph), 161.41 (CH=N).
MS (m/z, %): 261 (M +, 64.90), 149 (NO2Ph-C=N, 36.00 ), 126(C5H3NClN, 15.00 ), 112 (C5H3NCl, 34.00), 92( C5H3N2, 29.00 ), 77 ( C5H3N, 31.20), 53 (N=CH-CH=CH, 35.00), 51 (N=C-C=CH, 100.00).
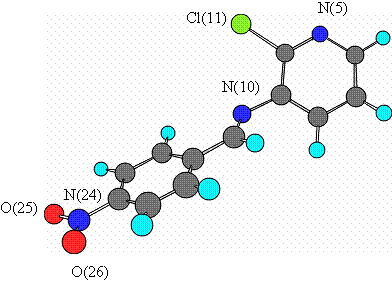
Figure 1 : The minimum energy conformation of Schiff base 3
The authors thank the Shiraz University Research Council for financial support (Grant No. 81-SC-1495-C191 ).
References
1. A..Shaikh kabeer , M.A.Baseer , N.A.Mote Asian J. Chem. 2001, 13(2), 496-500
2. S.N.Pandeya , D.Sriram , G.Nath , E.De Clercq Il Farmaco 1999, 54, 624-628
3. Afaf.H.El-masry , H.H.Fahmy , S.H.Ali Abdelwahed Molecules 2000, 5, 1429-1438
4. P.G.More , R.B.Bhalvankar , S.C.Pattar J. Indian Chem. Soc. 2001, 78, 474-475
5. W.M.Singh , B.C.Dash Pesticides 1988, 22 (11) , 33-37
6. S.B.Desai , P.B.Desai , K.R.Desai Heterocycl. Commun. 2001, 7 (1), 83-90
7. P.Phatak , V.S.Jolly , K.P.Sharma Orient. J. .Chem. 2000, 16 (3), 493-494
8. S.Samadhiya , A.Halve Orient. J. Chem. 2001, 17 (1), 119-122
9. Kristina Sepcic J. Toxicol. , Toxin Rev. 2000, 19 (2), 139-160
10. W.Sliwa , B.Mianowska Heterocycles 1989, 29 (3), 557-595
11. I.Druta , R.Dinica , E.Bacu , M.Anderi Chem. Abstr. 2000, 132, 205326b
Sample Availability : Available from MDPI.
© 2004 MDPI. All rights reserved.
¡¡