|
Molbank 2005, M393 |
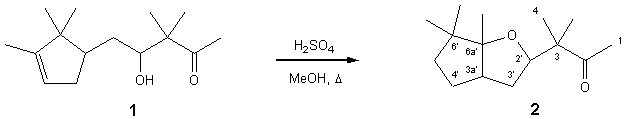
3-Methyl-3-(6,6,6a-trimethyl-hexahydro-cyclopenta[b]furan-2-yl)-butan-2-one
Juan M. Castro, Ram車n Porras, Pablo J. Linares-Palomino, Sof赤a Salido, Joaqu赤n
Altarejos,* Manuel Nogueras and Adolfo S芍nchez
Departamento de Qu赤mica Inorg芍nica y Org芍nica, Facultad de Ciencias
Experimentales,
Universidad
de Ja谷n, 23071 Ja谷n, Spain
Tel.: 34-953-002743, Fax: 34-953-012141,
e-mail: [email protected]
Received:
Keywords: Acid-catalyzed rearrangement, intramolecular cyclization, hexahydrocyclo-penta[b]furan derivative.
A 40% aqueous solution of sulfuric acid (0.5 mL) was added
to a stirred solution of 3,3-dimethyl-4-hydroxy-5-(2,2,3-trimethyl-3-cyclopentenyl)-pentan-2-one
(1) (630 mg, 2.65 mmol) in methanol (5 mL) and the mixture refluxed for 1.5 h. The resulting
solution was partially evaporated under reduced pressure, resolved in Et2O
and washed with saturated Na2CO3 (3℅25 mL) and brine (3℅25 mL). The
organic layer was dried over anhydrous Na2SO4 and
evaporated under reduced pressure to give a crude, which was filtered through a
silica gel pad to yield the cyclized and rearranged
title compound 2 (504 mg, 2.11 mmol, 80%) as a
yellow liquid.
IR (neat, cm-1): 1705 (CO), 1077, 906 (C-O-C).
1H NMR (300 MHz, CDCl3): 汛= 0.82 (3H, s, Me-6*); 0.96 (3H, s, Me*-6*); 1.03 (3H, s, Me-6a*); 1.05 (3H, s, Me-3); 1.09 (3H, s, H-4); 1.16-1.28 (1H, m, H-4*); 1.35 (1H, dd, J=12.2 Hz, 8.1 Hz, H-5*); 1.50-1.63 (2H, m, H-3*, H*-5*); 1.78-1.88 (1H, m, H*-3*); 1.90-2.06 (1H, m, H*-4*); 2.20 (3H, s, H-1); 2.31-2.42 (1H, m, H-3a*); 3.97 (1H, dd, J=11.5 Hz, 4.7 Hz, H-2*).
Some signals were assigned by means
of 2D NMR experiments.
13C NMR (75 MHz, CDCl3): 汛= 26.87 (C-1); 213.73 (C-2); 50.09 (C-3);
18.53 (C-4); 21.82 (Me-3); 84.68 (C-2*); 34.81 (C-3*); 46.84 (C-3a*); 29.33
(C-4*); 40.54 (C-5*); 46.43 (C-6*); 94.96 (C-6a*); 22.08 (Me-6*); 25.23
(Me*-6*); 19.60 (Me-6a*).
Some signals were assigned by means
of 2D NMR experiments.
EI-MS (70 eV,
m/z): 238 (M+, 0.2%); 223 (M+每Me, 0.4); 195 (M+每COMe, 0.1); 153 (M+每C5H9O,
15); 109 (10); 96 (22); 86 (47); 71 (13); 55 (13); 43 (CH3CO+,
100).
Acknowledgements
We wish to thank the Ministerio de Ciencia y Tecnolog赤a for financial support (R+D Project
PPQ2000-1665) and the Ministerio de Educaci車n, Cultura y Deporte for a
fellowship to J. M. Castro.
© 2005 MDPI. All rights reserved.