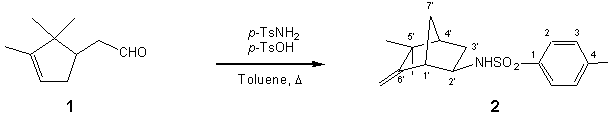|
Molbank 2005, M394 |
exo-N-(5,5-Dimethyl-6-methylene-bicyclo[2.2.1]hept-2-yl)-4-methyl-benzenesulfonamide
Concepci車n Bad赤a, Juan M. Castro, Pablo J.
Linares-Palomino, Sof赤a Salido, Joaqu赤n Altarejos,* Manuel Nogueras and Adolfo
S芍nchez
Departamento de Qu赤mica Inorg芍nica y Org芍nica,
Facultad de Ciencias Experimentales,
Universidad de Ja谷n, 23071 Ja谷n, Spain
Tel.:
34-953-002743, Fax: 34-953-012141, e-mail: [email protected]
Received:
Keywords: a-Campholenic aldehyde, acid-catalyzed rearrangement, intramolecular
cyclization.
p-Toluenesulfonic acid (35 mg, 0.20 mmol) was added to a stirred mixture of campholenic
aldehyde (1) (725 mg, 3.81 mmol)
and p-toluenesulfonamide (1.22 g, 6.96 mmol) in toluene (20 mL). Then a
Dean-Stark trap device was fit and the reaction refluxed for 0.5 h. After that,
the mixture was cooled to 0oC, filtered through a silica gel pad and
the solvent evaporated under reduced pressure to yield a residue (1.30g) which
was purified by flash chromatography on silica gel, using a 2:1 Hexane/Et2O
mixture as eluent, to give the title compound 2
(517 mg, 1.69 mmol, 44%).
Melting point:
110.7每114.8 oC (White crystals, from
hexane)
IR (KBr, cm-1):
3274 (N-H); 3063, 1663, 881 (C=C); 3062, 811 (Ar); 1327, 1164 (SO2);
666 (C-N).
1H NMR (300 MHz, CDCl3): 汛= 0.94 (3H, s, Mea-5*); 0.98 (3H, s, Meb-5*); 1.08 (1H, dt, J=13.7 Hz, 4.2 Hz, Hb-3*); 1.32 (1H, d, J=10.6 Hz, H-7*); 1.63
(1H, br d, J=10.6 Hz, H*-7*); 1.85 (1H, br d, J=2.3 Hz, H-4*);
2.14 (1H, ddd, J=13.7 Hz, 8.1 Hz, 2.5 Hz, Ha-3*); 2.41 (1H, br s, H-1*);
2.44 (3H, s, Me-4); 3.22 (1H, td, J=7.9 Hz, 4.2 Hz, H-2*); 4.59
(1H, s, CH2-6*); 4.72 (1H, s, CH2-6*); 4.87
(1H, d, J=7.9 Hz, N-H); 7.32 (2H, d, J=8.1 Hz, H-3, H-5); 7.77
(2H, d, J=8.1 Hz, H-2, H-6).
Some signals were assigned by means
of 2D NMR experiments.
13C NMR (75 MHz, CDCl3): 汛= 137.76 (C-1); 127.16 (C-2); 129.67
(C-3); 143.33 (C-4); 21.50 (Me-4); 52.82 (C-1*); 55.72 (C-2*); 35.23 (C-3*);
47.34 (C-4*); 41.00 (C-5*); 161.07 (C-6*); 34.02 (C-7*); 103.19 (CH2-6*);
25.30 (Mea-5*);
29.10 (Meb-5*).
Some signals were assigned by means
of 2D NMR experiments.
EI-MS (70 eV,
m/z): 305 (M+, 3%); 264 (2); 240 (M+每SO2H,
10); 201 (2); 185 (3); 184 (6); 155 (Ts+, 14); 150 (M+每Ts,
23); 134 (M+每TsNH2, 25); 121 (M+每TsNH每Me, 47); 108 (69); 91 (MePh+,
100); 79 (23); 65 (38); 53 (14); 41 (27).
Acknowledgements
We wish to thank the Ministerio de Ciencia y Tecnolog赤a for financial support (R+D Project
PPQ2000-1665) and the Ministerio de Educaci車n, Cultura y Deporte for a
fellowship to J. M. Castro.
© 2005 MDPI. All rights reserved.