|
Molbank 2005, M396 |
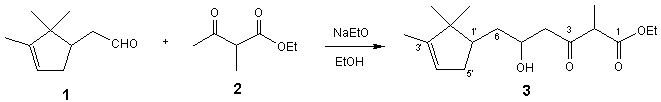
5-Hydroxy-2-methyl-3-oxo-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hexanoic acid ethyl ester
Mercedes P谷rez-Bonilla, Juan M. Castro, Pablo J. Linares-Palomino, Sof赤a
Salido, Joaqu赤n Altarejos,* Manuel Nogueras and Adolfo S芍nchez
Departamento de Qu赤mica Inorg芍nica y Org芍nica, Facultad de Ciencias
Experimentales,
Universidad de Ja谷n, 23071 Ja谷n, Spain.
Tel.:
34-953-002743, Fax: 34-953-012141, e-mail: [email protected]
Received:
Keywords: a-Campholenic aldehyde, ethyl 2-methylacetoacetate, aldol
reaction.
Sodium (241 mg, 10.48 mmol) was added to
absolute ethanol (40 mL) and the mixture refluxed until
sodium reacted completely. Then ethyl 2-methylacetoacetate (2) (1.2 mL,
8.25 mmol) was added to the solution. The mixture was
refluxed for 1 h and, then, after reaching room temperature, aldehyde 1 (1.26
g, 8.27 mmol) was added and the mixture refluxed
again for 2 h. Finally, ethanol was partially evaporated under reduced pressure
and the residue neutralized with aqueous 2N HCl and
extracted with EtOAc (3℅15 mL).
The combined organic layers were washed with 2N HCl
(10 mL), saturated NaHCO3 (2℅15 mL) and brine (3℅10 mL). The
organic phase was dried over anhydrous Na2SO4 and the
solvent evaporated under reduced pressure to yield a residue (1.65 g) which was
purified by flash chromatography on silica gel, using a 6.5:4.5 hexane/EtOAc mixture as eluent, to give
the title compound 3 (356 mg, 1.20 mmol, 15%).
Melting point: 131.0每132.8 ºC
(white dust, from hexane/ethyl acetate).
IR (neat, cm-1):
3450每2600, 1101 (OH); 3036 (C=C); 1610 (CO); 1610, 1246, 1153 (COOEt).
1H NMR (300 MHz, CDCl3): 汛= 0.79 (3H, s, Me-2*); 1.01 (3H, s, Me*-2*); 1.21 (3H, t, J=7.0 Hz, CH3CH2O), 1.36 (3H, d, J=6.6 Hz, Me-2); 1.62 (3H, br s, Me-3*); 1.70每2.87 (7H, m, H-4, H-6, H-1*, H-5*); 3.48 (2H, q, J=7.0 Hz, CH3CH2O); 3.61 (1H, q, J=6.6 Hz, H-2); 4.69每4.81 (1H, m, H-5); 5.24 (1H, br s, H-4*).
13C NMR (75 MHz, CDCl3): 汛= 169.92 (C-1); 51.68 (C-2); 201.52 (C-3); 43.98 (C-4); 73.19 (C-5); 35.25* (C-6); 7.71 (Me-2); 15.22 (CH3CH2O); 65.81 (CH3CH2O); 44.91 (C-1*); 46.79 (C-2*); 148.67 (C-3*); 121.00 (C-4*); 35.17* (C-5*); 19.73 (Me-2*); 25.46 (Me*-2*); 12.54 (Me-3*).
*These signals may be interchanged.
EI-MS (70 eV, m/z): 279 (M+每OH, 0.1%); 267 (M+每Et, 0.1);
251 (M+每OEt, 0.8); 235 (0.7); 217 (0.9);
205 (0.4); 193 (1); 171 (2); 161 (9); 141 (9); 133 (11); 119 (20); 108 (C8H12+,
100); 93 (29); 79 (12); 43 (34).
Acknowledgements
We wish to thank the Ministerio de Ciencia y Tecnolog赤a for financial support (R+D Project
PPQ2000-1665) and the Ministerio de Educaci車n, Cultura y Deporte for a
fellowship to J. M. Castro.
© 2005 MDPI. All rights reserved.