|
Molbank 2005, M399 |
Synthesis
of 4,6-bis(thiomorpholin-4-ylmethyl)-1,2,3-benzenetriol
A. Ma. Vel¨¢zquez1, L.Torres1,
R.Gonz¨¢lez1, A.Valencia1, I.Mart¨ªnez1,
A.Pecina1, I.Menconi1, L.Mart¨ªnez1, A.Ram¨ªrez1,
R.Hern¨¢ndez1, R.L¨®pez-Castañares2 , O.Olvera-Neria3,
E.Angeles*1,
1) Facultad de Estudios Superiores Cuautitl¨¢n, Universidad Nacional Aut¨®noma de M¨¦xico,
2) Facultad de Qu¨ªmica de la UAEM,
3) Instituto de Ciencias B¨¢sicas e Ingenieria, Universidad Aut¨®noma
e-mail: [email protected]
Received:
Keywords:
phenol, thiomorpholine
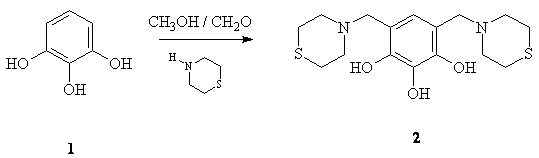
 4,6-bis(thiomorpholin-4-ylmethyl)-1,2,3-benzenetriol
(2) was prepared from pyrogalol (1) and
thiomorpholine and formaldehyde (37%) in methanol as solvent . A solution of
methanol (50 mL) and pyrogalol (1.24g, 9.83mmol) 1 was prepared,
after that a solution of thiomorpholine (2.0g, 20.70 mmol)
and formaldehyde (2.0 mL, 24.6 mmol)
in methanol were added at rt. When the addition was completed, the reaction
mixture was stirred at room temperature for 10 minutes and one red solid was
formed and it was isolated for filtration and recristalizaded
from ethanol (2.90g, 83% yield).
4,6-bis(thiomorpholin-4-ylmethyl)-1,2,3-benzenetriol
(2) was prepared from pyrogalol (1) and
thiomorpholine and formaldehyde (37%) in methanol as solvent . A solution of
methanol (50 mL) and pyrogalol (1.24g, 9.83mmol) 1 was prepared,
after that a solution of thiomorpholine (2.0g, 20.70 mmol)
and formaldehyde (2.0 mL, 24.6 mmol)
in methanol were added at rt. When the addition was completed, the reaction
mixture was stirred at room temperature for 10 minutes and one red solid was
formed and it was isolated for filtration and recristalizaded
from ethanol (2.90g, 83% yield).
Melting Point: 181-183 C¡ã (ethanol, uncorrected)
IR (CHCl3 film, cm-1): 3429 (O-H); 3065 (Csp2-H Ar); 2872 (Csp3-H).
1H-NMR (300 MHz; CDCl3): ¦Ä= 8.40 (3H, s, OH); 6.20 (1H, s); 3.61 (4H, s, Ar-CH2); 2.81 (8H, m, -S-CH2-); 2.72 (8H, m, -N-CH2-); 1.27.
13C-NMR (75 MHz; CDCl3): ¦Ä= 144.8 (C); 132.51 (C); 118.42 (CH); 111.74(C); 61.63 (Ar-CH2); 54.22 (-N-CH2-); 27.81 (-S-CH2-).
MS (FAB; m/z, %): 357 (10%); 254(100%); 102(91).
Elemental Analysis: Calculated for C16H24N2O3S2:
C, 53.91%; H, 6.79%; N, 7.86%; O, 13.46%; S,17.99%. Found: C, 53.90%; H, 6.74%;
N, 57.91%; O, 13.37%; S, 18.02%.
Acknowledgements
The authors wish to acknowledge to PAPIIT/UNAM Projects No IN205902 and IN207705 and ALPHARMA SA de CV, by partially support this work. We would like to thank C.Barajas, F.Sotres, P.Garc¨ªa, D.Jim¨¦nez from FESC-UNAM and Rosa I.del Villar M., Oscar Yañez and Georgina Duarte from USAI-UNAM for their skillful technical assistance and DGSCA-UNAM for their support. As a part of Project C¨¢tedra Qu¨ªmica Medicinal of FESC-UNAM.
References
 1. Artico, M.; Mai, A.; Sbardella, G.; Massa, S.; Lampis, G.; Deidda, D.; Pompei, R. Bioorganic
& Medicinal Chemistry Letters
1998, 8, 1493-1498.
1. Artico, M.; Mai, A.; Sbardella, G.; Massa, S.; Lampis, G.; Deidda, D.; Pompei, R. Bioorganic
& Medicinal Chemistry Letters
1998, 8, 1493-1498.
2. Biava, M.; Fioravanti,
R.; Porretta, G.C.; Deidda,
D.; Maullu, C.; Pompei, R. Bioorganic
& Medicinal Chemistry Letters
1999, 9, 2983-2988.
3. Mai, A.; Sbardella,
G.; Artico, M.; Massa, S.; Lampis, G.; Deidda, D.; Pompei, R. Medicinal Chemistry Research 1999, 9, 149-161.
© 2005 MDPI. All rights reserved.