|
Molbank 2005, M408 |
4-Methyl-N-(2,2,4,4-tetrachloro-3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide
Holger Meining and Baldur Föhlisch*
Institut f邦r Organische Chemie der Universität Stuttgart,
Pfaffenwaldring 55, D-70569 Stuttgart, Germany
Fax: (+ 49) 711/6854269; e-mail: [email protected]
Received:
Keywords: cycloaddition, ketones, sulfonamides, heterocycles
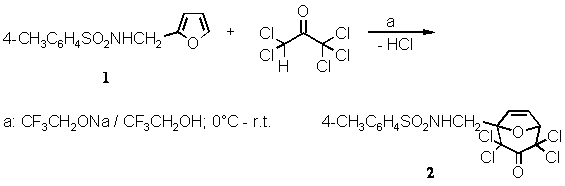
A mixture of N-furfuryl-p-toluenesulfonamide (1) (2.51 g, 10 mmol) [1] and pentachloroacetone [2] (3.23 g, 14 mmol) was cooled in an ice bath. With magnetic stirring, a 2-molar solution of sodium 2,2,2-trifluoroethoxide in 2,2,2-trifluoroethanol[3] (7 mL, 14 mmol) was added dropwise, over 15 min. Stirring was continued for 15 min at 0 ∼C and then at room temperature for 2每3 hours[4]. The mixture was allowed to stir for a further 2 hours. Then, the heterogeneous mixture was poured on saturated brine (20 mL). The precipitate was dissolved by adding a little of dichloromethane and water, and the organic layer was separated. The aqueous layer was acidified with hydrochlorid acid to pH 4每5 and then extracted with dichloromethane (4 ´ 20 mL). The combined dichloromethane solutions were washed with saturated brine (20 mL) and dried overnight with magnesium sulfate. After filtration, the solution was concentrated in a rotary evaporator. The remaining yellow mass was recrystallized from dry ethanol (10 mL) to yield 3.60 g (81%) of 2 as a colourless crystalline solid.
Melting Point: 162每163 ∼C.
TLC (silica, hexane/tert-butylmethyl ether (1:1 v/v): A pale yellow spot emerged after spraying the sheet with vanillin/sulfuric acid reagent followed by heating with a hot-air gun; Rf = 0.27. The starting material (1) showed a brownish violet spot at Rf = 0.37.
1H-NMR (250 MHz, CDCl3): 汛= 2.45 (s, 3 H,
CH3C6H4SO2);
ABX sub-spectrum (14 lines) with 汛 A = 3.89, 汛 B = 3.65,
汛 X = 5.10, JAB
= (-)14 Hz, JAX = 9.6 Hz, JBX = 3.6 Hz, 3 H, diastereotopic CH2-NH); 5.16 (d, J = 1.7 Hz, 1 H, H-5), AB sub-spectrum
with 汛 A = 6.48 (H-6), 汛 B = 6.46 (H-7), JAB = 5.5 Hz, the lines of
the A part are split into doublets with J
= 1.7 Hz; AA*BB* sub-spectrum with 汛 A = 7.76, 汛 B =
7.35, JAB = 8.2 Hz (H-2/6
and H-3/5 from CH3C6H4SO2).
13C-NMR/DEPT (62.9 MHz, CDCl3): 汛= 21.6
(+, CH3C6H4SO2);
42.2 (-, CH2-N); 81.9 (Cq,
C-4); 85.05 (Cq, C-2); 87.9 (+, C-5); 93.3
(Cq, C-1); 127.0 (+, C-2/6 of the tosyl group); 130.0 (+, C-3/5 of the tosyl
group); 134.4 (+, C-6); 135.7 (+, C-7); 136.7 (Cq,
C-4 of the tosyl group); 144.1 (Cq,
C-1 of the tosyl group); 183.9 (Cq,
C-3).
IR (CHCl3 film, cm-1): 3395, 3280 (N-H); 3110 (=C-H); 1768 with shoulder at 1750 (C=O);
1600 (C=C); 1495 (NH); 1430, 1340 (SO2); 1163 cm-1 (SO2).
Elemental
Analysis: Calculated for C15H13Cl4NO4S
(445.15): C, 40.47%; H, 2.94%; Cl, 31.86%; N: 3.15%;
S, 7.20%. Found: C, 40.24%; H, 3.02%; Cl, 31.63%; N,
3.15%; S, 7.15%.
References and Notes
1. a) Chooney, N.; Kuhnert, N.; Sammes, P. G.; Smith, G.; Ward, R. W. J. Chem. Soc., Perkin Trans 1, 2002, 1999每2005. b) Meining, H.; Föhlisch, B. Molbank 2005, M407.
2. Bugrova,
L. V.; Rudnev, G. K.; Kristich,
A. I.; Radchenko, V. I.; Mishchenko,
L. F. Zh. Prikl. Khim. (Leningrad) 1973,
46, 1529每1533; J. Appl. Chem. USSR 1973,
2, 1627每1631.
3. a) Föhlisch, B.; Gehrlach, E.; Herter, R. Angew. Chem. 1982, 94, 144; Angew. Chem. Suppl. 1982, 94, 241; Angew. Chem., Int. Ed. Engl. 1982, 21, 137. b) Sendelbach, S.; Schwetzler-Raschke, R.; Radl, A.; Kaiser, R.; Henle, G. H.; Korfant, H.; Reiner, S.; Föhlisch, B. J. Org. Chem. 1999, 64, 3398每3408.
4. If 1 has not disappeared after that time (check by TLC), more pentachloroacetone (0.7每1.2 g, 3每5 mmol) was added, and the base solution in such amount that a test with wet pH indicator paper showed an alkaline reaction.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.