|
Molbank 2005, M410 |
4-Methyl-N-(3-oxo-8-oxabicyclo[3.2.1.]oct-6-en-1-ylmethyl)-benzenesulfonamide
Holger Meining and
Baldur Föhlisch*
Institut f¨¹r
Organische Chemie der Universität Stuttgart,
Pfaffenwaldring
55, D-70569 Stuttgart, Germany
Fax: (+ 49) 711/6854269; e-mail: [email protected]
Received:
Keywords: dehalogenation, ketones, sulfonamides, heterocycles
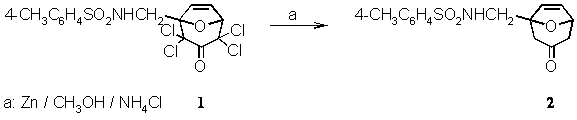
Zinc powder (10.6 g, 160 mmol) was given to an ice-cold solution of methanol (40 mL) saturated with ammonium chloride. With vigorous magnetic stirring in an ice-bath, tetrachloroketone 1[1] (4.45 g, 10 mmol) was added in small portions (exothermic reaction, the mixture should not be allowed to boil!). Then the ice-bath was removed, and the mixture was allowed to come up to room temperature with continuous stirring. Finally, the mixture was refluxed. A TLC showed that after 1 h the starting material 1 had disappeared. Unreacted zinc powder and inorganic salts were removed by suction and washed with methanol (50 mL). To the combined filtrates, 100 mL of a 0.6 molar solution of EDTA disodium salt (110 g in 500 mL water with 10 g NaOH) was added. The mixture was stirred for 1 h and extracted with tert-butylmethyl ether (9 ´ 20 mL). The combined extracts were washed with saturated brine (50 mL) and dried overnight with magnesium sulfate. After filtration the solvent was evaporated. The remaining solid had a slight pink colour. It was crystallized from a mixture of ethanol and tert-butylmethyl ether (1:1 v/v). The pink needles were filtrated and washed with n-pentane to give 2.71 g (88% yield)
Melting Point: 106¨C107 ¡ãC.
TLC (silica, hexane/tert-butylmethyl ether (1:1 v/v): A yellow spot emerged after spraying the sheet with vanillin/sulfuric acid reagent followed by heating with a hot-air gun; Rf = 0.05. The starting material (1) showed a pale yellow spot at Rf = 0.27.
1H-NMR (250 MHz, CDCl3): ¦Ä= 2.43 (s, 3 H, CH3C6H4SO2) AB sub-spectrum with ¦ÄA = 2.55, ¦ÄB = 2.29, JAB= 16.3 Hz (2 H, H-2x and H-2n); AB sub-spectrum with ¦ÄA = 2.66, ¦ÄB = 2.31, JAB= 16.5 Hz, the lines of the A part are split into a doublet with J= 5.0 Hz (2 H, H-4x and H-4n); ABX sub-spectrum with ¦ÄA= 3.31, ¦ÄB= 3.17, ¦ÄX= 5.15, JAB= (-)13.1 Hz, JAX= 7.5 Hz, JBX= 4.8 Hz (3 H, diastereotopic CH2-NH); 5.02 (d, J= 5.0 Hz, 1 H, H-5); 6.06 (d, J= 6.0 Hz, 1 H, H-7); 6.22 (dd, J= 6.0 Hz, J= 1.6 Hz, 1 H, H-6); AA¡¯BB¡¯ sub-spectrum with ¦ÄA= 7.74, ¦ÄB= 7.32, JAB= 8.2 Hz (4 H, H-2/6 and H-3/5 from CH3C6H4SO2).
13C-NMR (62.9 MHz, CDCl3): ¦Ä= 21.5 (CH3C6H4SO2); 45.2 (C-2); 47.0 (C-4); 48.4 (CH2-N); 77.7 (C-5); 85.3 (C-1); 127.0 (C-2/6 of the tosyl group); 129.8 (C-3/5 of the tosyl group); 133.4 (C-6); 134.9 (C-7); 136.6 (C-4 of the tosyl group); 143.6 (C-1 of the tosyl group); 204.5 (C-3).
IR (CHCl3 film, cm-1): 3370, 3280 (NH); 2970, 2925, 2830 (CH); 1720 (C=O); 1600 (C=C); 1495, 1455, 1410, 1340, 1165.
Elemental Analysis: Calculated for C15H17NO4S (307.4): C, 58.61%; H, 5.57%; N, 4.56%; S, 10.43%. Found: C, 58.73%; H, 5.55%; N, 4.48%; S, 10.59%.
References
1. Meining, H; Föhlisch, B. Molbank 2005, M409.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.