|
Molbank 2005, M415 |
Synthesis of 5-(2-aminoethoxy)-3-methyl-1-phenyl-1H-pyrazolo[4,3-E][1,2,4]triazine
Mariusz Mojzych
Institute of Chemistry University of Podlasie
ul. 3 Maja 54,
08-110 Siedlce, Poland
e-mail: [email protected]
Received:
Keywords: 1H-pyrazolo[4,3-e][1,2,4]triazine,
2-aminoethanol, nucleophilic
substitution.
A variety of naturally occurring O-
and N-derivatives of 1H-pyrazolo[4,3-e][1,2,4]triazine exhibit an interesting combination of biological
activity [1-2]. As a part of our ongoing research programme [3-6] we have
synthesised a novel 5-(2-aminoethoxy)- 3-methyl-1-phenyl-1H-pyrazolo[4,3-E][1,2,4]triazine derivative of this ring system based on nucleophilic
displacement of methylsulfonyl group [6].
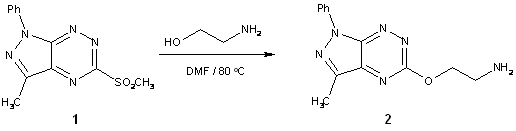
To a solution of sulfone
1 (145 mg, 0.5 mmol)
in anhydrous DMF (5 ml) 2-aminoethanol (0.5 ml) was added and the resulting
mixture was stirred at 80 ºC for 2 h. After pouring onto cold water
the precipitate was filtered off, washed with water and recrystallized
from ethyl alkohol to give 5-(2-aminoethoxy)-3-methyl-1-phenyl-1H-pyrazolo[4,3-e][1,2,4]triazine (2) in 90% yield.
Melting
Point: 164 ºC.
1H-NMR (200 MHz, CDCl3): ¦Ä=
2.61 (s, 3H); 3.76-3.81 (m, 2H); 3.96 (t, 2H, J=6.0 Hz); 6.10 (s, 2H);
7.29-7.33 (m, 1H); 7.48-7.56 (m, 2H); 8.26-8.31 (m, 2H).
IR (KBr, cm-1):
3243; 2948; 1580; 1540; 750; 689.
MS (EI, 70eV; m/z, %): 270 (68) [M+]; 242 (4);
211 (60); 184 (14); 131 (20); 104 (42); 77 (100).
Elemental Analysis: Calculated for C13H14N6O: C,
57.77%; H, 5.18%; N, 31.11%. Found: C, 57.67%; H, 5.17%; N, 31.16%.
References:
1. Hirata, K.; Nakagami,
H.; Takashina, J. et. al., Heterocycles, 1996, 43, 1513.
2. Smirnov, V.V.; Kiprianowa,
E.A. et. al., FEMS
Microbiology Lett., 1997, 153, 357.
3. Rykowski,
A.; Mojzych, M.; Karczmarzyk,
Z., Heterocycles,
2000, 53, 2175.
4. Karczmarzyk,
Z.; Mojzych, M.; Rykowski,
A., J. Chem. Cryst.,
2000, 30, 423.
5. Mojzych,
M.; Rykowski, A., Polish
J. Chem., 2003, 77, 1797.
6. Mojzych,
M., Rykowski, A., Heterocycles, 2004, submitted.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights
reserved.