|
Molbank 2005, M424 |
3,8-Diethynyl-[1,10]-phenanthroline
Davood Habibi
Department of Chemistry, Faculty of Sciences, Bu
e-mail: [email protected]
Received:
Keywords: 3,8-disubstituted-[1,10]-phenanthroline,
Eaborn procedure, hydrolysis
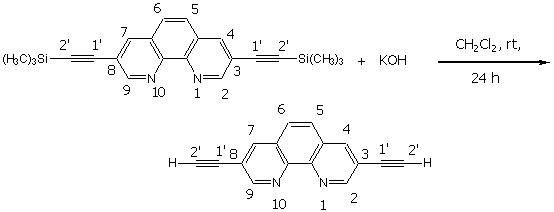
The experimental procedure follows a protocol developed by Eaborn [1]. To a solution of 3,8-bis-trimethylsilanylethynyl-[1,10]-phenanthroline (372.5 mg, 1.0 mmol), dissolved in THF (20 mL), was added 1 M KOH in methanol (20 mL). The resultant solution was stirred for 24 hours at room temperature. After addition of water (50 mL), the product was extracted with CH2Cl2 (3x 30 mL). Removal of the solvent in vacuo afforded 228 mg (1.0 mmol) of 3,8-diethynyl-[1,10]- phenanthroline (100%) as a colorless solid.
Melting Point: ~ 295-300 ¡ãC.
IR (KBr, cm-1): 3140, 2086, 1588, 1551, 1499, 1418, 1264, 1222, 1096, 904, 838, 729.
1H-NMR (250 MHz, CDCl3,): ¦Ä= 3.0 (2H, 2'-H); 7.5 (2H, 5-H & 6-H); 8.2 (2H, 4-H & 7-H); 9.0 (2H, 2-H & 9-H).
Elemental Analysis: Calculated for C16H8N2: C, 84.19%; H, 3.53%; N, 12.27%. Found: C, 84.0%; H, 3.4%; N, 12.5%.
Acknowledgements
The author gratefully acknowledges
the financial supports from the Bu Ali Sina
University,
References
1. Eaborn, C.; Walton, D. R. M. J. Organometal. Chem. 1965, 4, 217.
© 2005 MDPI. All rights reserved.