|
Molbank 2005, M425 |
Synthesis of
methyl N'-[1-(4-hydroxybutyl)-3-(trifluoromethyl)-1H-1,2,4-triazol-5-yl]-N-phenylimidothiocarbamate
Abdelfattah Haikal*
and Hussein F. Zohdi
Department
of Chemistry, Faculty of Science,
e-mail: [email protected]
and [email protected]
Received:
Keywords: Synthesis, triazol, trifluoromethyl
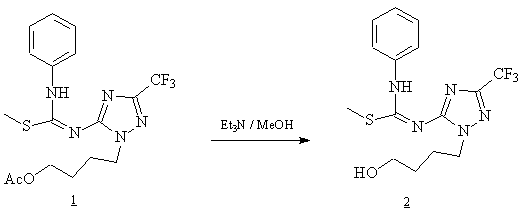
The desired compound 2 was obtained by complete deacetylation of compound 1 [1] using triethylamine [2]. To a solution of 1 (0.623g, 1.5 mmol) in methanol (15 ml) was added triethylamine (2 ml). The mixture was stirred at room temperature and the reaction was followed by TLC. After complete deacetylation (24 hours), the reaction mixture was evaporated and coevaporated with methanol (3x30 ml) under reduced pressure, then chromatographed over silica gel using CH2Cl2/MeOH (98:2 v/v) to give compound 2 as white powder crystallized from n-Hexane/ Ethylacetate.
Melting point: 113-114 ºC.
Elemental Analysis: Calculated for C15H18F3N5SO: C, 48.26%; H, 4.83%; N, 18.77%. Found: C, 48.35%; H, 4.98%; N, 18.75%.
UV (EtOH, ¦Ëmax): 277nm
IR (KBr, cm-1): 3448 (OH group)
MS (m/z): 374 (M+1)
1H-NMR (250 MHz, DMSO): ¦Ä= 1.7(m,2H,HOCH2CH2); 2.1(m,2H, CH2CH2-N); 2.1 (s,3H,SCH3); 3.7(t,2H,OCH2 ); 4.3 (m,2H,NCH2); 4.8(m,1H.OH, D2O exchangeable); 7.6-7.8 (m,5H, aromatic).
References
1. Haikal, A.; Zohdi, H.; submitted to Molbank 2003.
2. Zohdi, H.; Haikal, a.; Molecules 2001, 6, M263.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.