|
Molbank 2005, M426 |
1-Hydroxymethyl-3,5-diphenylpyrazole
Ibrahim Bouabdallah, Abdelkrim Ramdani*, Ismail Zidane and Rachid Touzani
Laboratory of Physical Organic
Chemistry, Department of Chemistry, Faculty of Sciences, University Mohammed
the first
BP 524, 60 000
e-mail : Bouabdallah@sciences.univ-oujda.ac.ma, [email protected]
Received:
Keywords: pyrazol, formaldehyde
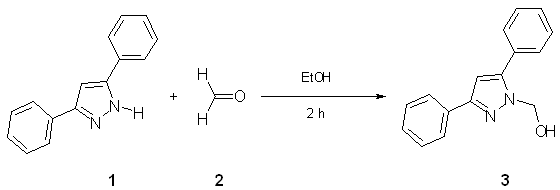
To a solution of 3,5-diphenylpyrazole 1 [1] (200 mg, 0.90 mmol)
in ethanol (10 mL) was added formaldehyde 2 (110 mg, 1.28 mmol,
35 %) and the mixture was refluxed for two hours [2, 3]. The reaction was
continued at room temperature for 12 h. The mixture was concentrated at reduced
pressure. The residue was purified by recrystallization
to afford the product 3 as a white
solid.
Yield: 160 mg, 71 %.
Melting point: 122-123¡ãC (CH3CH2OH).
IR (KBr, cm-1): 3120 (¦ÍO-H); 1530 (¦ÍC=C);
1440, 1423, 1380, 1360, 1260 (¦ÄO-H); 1180, 1150,
1131, 1030, 1010, 927, 905, 760, 690.
1H-NMR (200 MHz, CDCl3):
¦Ä= 7.89 (m, 2 H, CH ph : o-H); 7.74 (m, 2 H, CH ph : o-H); 7.55
(m, 3 H, CH ph : m-H, p-H); 7.49 (m, 3 H, CH ph : m-H, p-H); 6.75
(s, 1H, CH pz); 5.70 (s, 2 H, CH2).
MS (EI, 70eV; m/z): 250; 220; 191; 165; 143; 126; 110; 89; 77; 63;
51; 40.
References and Notes:
1.Ali,
S. S.; Ashraf, C. M. ; Younas, M.
and Ehsan, A.
Pak. J. Sci.
Ind. Res., 1993, 36, 12, 502-510.
2.Driessen, W. J.
R.. Neth. Chem. Soc. 1982, 101, 441.
3.Bouabdallah,
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights
reserved.