|
Molbank 2005, M428 |
Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)benzene and Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)diphenyl
Milan C. Dutta, Kaushik Chanda, Kaushal Kishore, J.N.
Vishwakarma*
Organic Research Lab, Department of Chemistry, St. Anthony¡¯s
College,
Shillong-793 001 (
Phone: 0364.2229928, Fax: 0364.2229558, e-mail: [email protected]
Received:
Keywords: nitroketene S, N-acetal, cyclocondensation, 1,2,3,4-tetrahydropyrimidine.
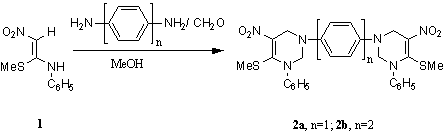
In
continuation with our on going program on the synthesis of tetrahydropyrimidines
[1, 2], we have recently reported the synthesis of Bis-tetrahydropyrimidines
[3] in which the rings are linked through flexible aliphatic chains. We now
report the synthesis of the title compounds in which the rings are bonded
through rigid aromatic systems. A mixture of p-phenylenediamine
(54 mg, 0.5 mmol) and formaldehyde (60 mg, 2 mmol,
40% solution) was stirred in methanol (3 mL) for ten
minutes and to this a solution of 1-nitro-2-anilino-2-methylthioethene 1
[4] (210 mg,
1 mmol) in 6 mL methanol
was added and the mixture was stirred at room temperature for 5 hours, when a
yellow solid precipitated out. After the completion of the reaction (monitored
by tlc) the reaction mixture
was cooled in ice water and the solid filtered, washed with methanol (2X2 mL)
to give pure 2a (220 mg, 76%), which was recrystallized
from methanol. The reaction of 1 with benzidine
was carried out in refluxing methanol to give 2b in 63 % yield, which
was recrystallized from benzene.
1,4-Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)benzene
(2a)
Melting
point: 168-169 ºC (methanol, uncorrected).
IR (KBr,
cm-1): 1456; 1503; 1544; 1611.
1H-NMR (300 MHz, CDCl3): ¦Ä= 1.94 (s, 6H, SCH3);
4.49(s, 4H, N-CH2C=); 4.87 (s, 4H, N-CH2-N); 6.70-6.73
(m, 4H); 7.06-7.33 (m, 10H).
13C-NMR
(75 MHz, CDCl3): ¦Ä= 16.5;
48.8; 72.1; 116.5; 124.8; 126.4; 127.3; 129.8; 133.2; 145.5; 146.0; 160.2.
MS (m/z): 577
(M+).
4,4¡ä-Bis-(1-phenyl-5-nitro-6-methylthio-1,2,3,4-tetrahydropyrimidinyl)diphenyl (2b)
Melting point: 167-169
ºC (benzene,
uncorrected).
IR (KBr, cm-1): 1430; 1514; 1540; 1591.
1H-NMR (300 MHz, CDCl3): ¦Ä= 1.94 (s, 6H, SCH3);
4.49 (s, 4H, N-CH2C=); 4.87 (s, 4H, N-CH2-N); 6.70-6.73
(m, 4H); 7.14-7.36 (m, 14H).
13C-NMR (75 MHz, CDCl3): ¦Ä= 16.5; 48.8; 72.1;
116.5; 124.8; 126.4; 127.3; 128.3; 129.8; 133.2; 145.5; 146.0; 160.2.
MS (m/z): 653
(M+).
Acknowledgements:
The
authors wish to thank Fr. I. Warpakma for facilities
and Fr. Stephan Mavely & Fr. J. Nellanatt for encouragement. Financial assistance from
ICAR-NATP is gratefully acknowledged. The authors also wish to express their
gratitude to Dr. Anubrata Das
of ICAR for his keen interest in this investigation.
References:
1.Vishwakarma, J.N.; Mofizuddin,
M.; Ila, H.; Junjappa, H. J. Heterocyclic Chem. 1988,
25, 1387.
2.Karim, E.; Kishore, K.; Vishwakarma, J.N.
J. Heterocyclic Chem. 2003, 40, 901-03.
3.Chanda, K.; Dutta, M.C.; Kishore, K.; Vishwakarma, J.N. Molbank, 2004, M367.
4.Gompper, R.; Schaefer, H. Chem. Ber.
1967, 100, 591-604.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights
reserved.