|
Molbank 2005, M430 |
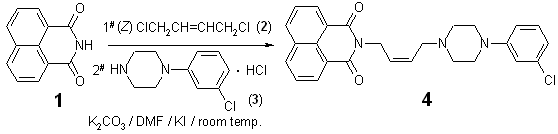
N-{(2Z)-4-[4-(3-chlorophenyl)piperazin-1-yl]but-2-enyl}-1,8-naphthalimide
Teresa Kowalska, Piotr Kowalski*
Institute of Organic Chemistry and
Technology,
24 Warszawska Str., 31-155
Phone (+4812)-628-27-22, e-mail: [email protected]
Received:
Keywords: arylpiperazines, serotonin receptors ligands
In continuation of our interest on synthesis and
properties of arylpiperazine derivatives investigated
as a serotonin receptors ligands [1-3], herein we
presented one-pot synthesis of N-{(2Z)-4-[4-(3-chlorophenyl)piperazin-1-yl]but-2-enyl}-1,8-naphthalimide
(4). Starting materials e.g. 1,8-naphthalimide (1), (Z)-1,4-dichloro-2-butene (2),
and 1-(3-chlorophenyl)piperazine hydrochloride (3) were commercially available
reagents.
A
mixture of the 1,8-naphthalimide (1) (
Melting
point: 138每140 ºC. (for HCl salt mp 279每281 ºC)
1H-NMR (80 MHz, CDCl3): 汛= 2.64每2.76 (m, 4H,
2CH2-pip); 3.17每3.30 (m, 4H, 2CH2-pip); 3.39 (d, 2H, CH2-N-pip);
4.87 (d, 2H, CH2-N-imide); 5.68每5.79 (m, 2H, CH=CH); 6.74每7.26 (m,
4H, ArH); 7.74 (t, 2H, H3,6-imide); 8.22
(d, 2H, H4,5-imide); 8.61(d, 2H, H2,7-imide).
IR (KBr, cm-1):
2954-2780 (CH); 1699, 1663 (C=O); 1590-1435 (C=C).
MS (EI, 70 eV; m/z, rel.%):
445 [M+] (21); 250 (61); 235 (100); 196 (15); 180 (32).
Elemental
Analysis: Calculated for C26H24ClN3O2.HCl
(482.41): C, 64.73%; H, 5.22%; N, 8.71%. Found: C, 64.79%; H, 5.33%; N, 8.48%.
References
1. Kowalski, P.; Mokrosz, M. J.; Majka, Z.; Kowalska, T.; Duszy里ska, B. J. Heterocyclic Chem. 2000, 37, 187-189.
2. Kowalski, P.; Kowalska, T.; Mokrosz, M.J.; Bojarski, A.J.; Charakchieva-Minol,
S. Molecules 2001, 6, 784-795.
3. Bojarski,
A.J.; Kowalski, P.; Kowalska, T.; Duszy里ska,
B.; Charakchieva-Minol, S.; Tatarczy里ska,
E.; Kłodzi里ska, A.; Chojnacka-W車jcik,
E. Bioorg. Med. Chem. 2002, 10, 3817-3827.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.