|
Molbank 2005, M433 |
3-Nitropyridin-2-yl hydrazone of 5-Acetyl-3-phenyl-1,2,4-triazine
Mariusz Mojzych
Institute of Chemistry University of Podlasie
ul. 3 Maja 54, 08-110
Siedlce, Poland
e-mail: [email protected]
Received:
Keywords: hydrazone, pyridin-2-yl hydrazine,
5-acetyl-1,2,4-triazine.
In continuation
of previous work on the polyfunctionally substituted
1H-pyrazolo[4,3-e][1,2,4]triazine [1,2] we have reported here preparation of
3-nitropyridin-2-yl hydrazone of 5-acetyl-3-phenyl-1,2,4-triazine 3 being valuable intermediate for the
synthesis of nitro derivative of this ring system.
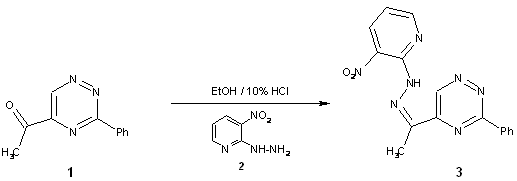
To a
solution of 5-acetyl-3-phenyl-1,2,4-triazine 1(199 mg, 1mmol)[3] and 3-nitropyridin-2-ylhydrazine 2 (185 mg, 1.2 mmol)
in ethanol (30 ml) 10% HCl (0.5 ml) was added. The resulting reaction mixture was
heated at reflux for 5 min and then was stirred at room temperature for 30 min.
After that time the solvent was concentrated in vacuo. The solid was collected by
filtration, washed with water and recrystallized from
DMSO/water (1:1) to give compound 3
in 82% yield.
Melting Point: 255ºC.
1H-NMR (200 MHz,
d6-DMSO): ¦Ä= 2.53 (s, 3H); 7.21-7.27 (m, 1H); 7.62-7.67 (m, 3H); 8.44-8.54 (m,
3H); 8.65-8.68 (m, 1H); 9.56 (s, 1H); 11.36 (s, 1H).
IR (CHCl3
film, cm-1): 3316; 3069; 1598; 1530; 1500; 1366; 1264; 1199; 1053;
754; 697.
MS-EI (m/z,
%): 335 (1) [M+]; 189 (30); 180 (9); 179 (100); 157 (6); 143 (8);
133 (5); 128 (7); 103 (7); 102 (4); 77 (5).
HR-MS: Calculated
for C16H13N7O2:
335.11307. Found: 335.11339.
References:
1.Rykowski, A.; Mojzych, M.; Karczmarzyk, Z. Heterocycles, 2000, 53, 2175.
2.Mojzych, M.; Rykowski A. Polish J. Chem., 2003, 77, 1797.
3.Rykowski, A.; Lipi¨˝ska, T. Synth. Comm., 1996, 26, 4409.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.