|
Molbank 2005, M438 |
Synthesis of
(4-chlorobenzylidene)-(2-chloropyridi-3-yl)amine
A. A. jarrahpour*
and M. Zarei
Department
of Chemistry,
Tel. 0098
711 2284822, Fax:
0098 711 2280926, e-mail: [email protected], [email protected]
Received:
Keywords: Schiff bases , 3-amino-2-chloropyridine , p-chlorobenzaldehyde , biological activity .
Schiff bases are widely in use for synthetic purposes both by organic and inorganic chemists.1 They are used as biological, analytical, polymer and liquid crystalline materials.2 Schiff bases are reported to show a variety of biological activities such as antibacterial3-5, antifungal6-7, anticancer 8-9 and herbicidal 10 activities . Pyridinium compounds have biological activities11 such as antifungal12 and antibacterial12-13 activities. The presence of a chloro moiety in different types of compounds causes them to exhibit pesticidal activity.10
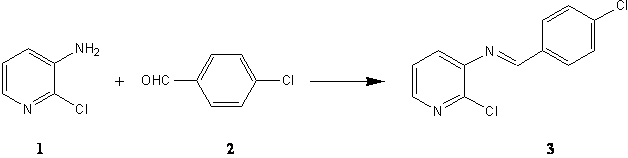
A mixture of 3-amino-2-chloro pyridine 1 (1.28 g, 10 mmol), 4-chlorobenzaldehyde 2 (1.40 g, 10 mmol) and anhydrous sodium sulfate (4.00 g) in dry dichloromethane (40.00 mL) was stirred at room temperature for seven hours. The suspension was filtered and washed with dichloromethane. The solvent was evaporated and new schiff base 3 was formed, which was washed with 10 mL acetic acid 5%, 10 mL NaHCO3 5% and water, successively, (2.04 g, 82 %).
Melting point: 54-56oC.
IR (KBr, cm-1): 1600.1 (CH=N); 1624.0 (C=N pyridine ring).
1H-NMR (250MHz, CDCl3): ¦Ä= 6.97-8.17 (7H, m, 2 Ph); 8.31 (1H, s, HC=N).
13C-NMR (62.9 MHz, CDCl3): ¦Ä= 123.55-146.49 (aromatic carbons);
162.55 (HC=N).
MS (m/z,
%): 251 ([M+],
63.3); 250 (M-1, 100.0); 215 (C5H3NClN=CC6H4
, 14.8); 139 (C5H3NClN=C, 15.4);
112 (C5H3NCl, 52.2 ), 89 (C5H3NN,
19.4); 77 (C5H3N, 15.1).
Acknowledgment
The
authors thank the Shiraz University Research Council for financial support
(Grant No. 83-GR-SC-31and 84-GR-SC-23 ).
References
1. K. Arora,
A. Gupta,
2. K. Tanaka, R. Shiraishi
Green Chem. 2000, 2, 272.
3. A. .Shaikh kabeer, M. A. Baseer, N. A. Mote Asian J. Chem. 2001,
13(2), 496.
4. S. N. Pandeya,
D. Sriram, G. Nath, E. De Clercq Il Farmaco 1999
,54 , 624.
5. Afaf. H.
El-masry, H. H. Fahmy, S.
H. Ali Abdelwahed Molecules 2000, 5,
1429.
6. P. G. More, R. B. Bhalvankar, S. C. Pattar J.
Indian Chem. Soc. 2001, 78, 474.
7.
W. M. Singh, B. C. Dash Pesticides 1988, 22 (11), 33.
8.
S. B. Desai, P. B. Desai, K. R. Desai Hetrocycl. Commun. 2001, 7 (1), 83.
9. P. Phatak , V. S. Jolly, K. P.Sharma Orient. J. Chem. 2000, 16 (3), 493.
10.
S. Samadhiya, A. Halve Orient. J. Chem. 2001,
17 (1), 119.
11. Kristina Sepcic J. Toxicol., Toxin Rev. 2000, 19 (2), 139.
12.
W. Sliwa ,
B. Mianowska Hetrocycles
1989, 29 (3), 557.
13. I. Durta, R. Dinica, E. Bacu, M. Anderi Chem.
Abstr. 2000, 132, 205326b.
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.