|
Molbank 2005, M446 |
10,14-Dibenzyl-6,18-dimethyl-1,5,10,14,19,20-hexaaza-tricyclo[14.2.1.15,8]eicosa-6,8(20),16(19),17-tetraen-12-ol
Rachid Touzani,a
Abdelkrim Ramdania
et Sghir El Kadirib*
aLaboratoire de Chimie
Organique-Physique
b*Laboratoire de Chimie de
l`Environnement et des Mat¨¦riaux
D¨¦partement
de Chimie, Facult¨¦ Des Sciences, Universit¨¦ Mohamed Premier,
60000
Oujda, Maroc
e-mail: [email protected]
Received: Received:
Keywords: macrocyclic compounds, macrocyclic cavity, pyrazole
Macrocyclic chemistry is one of
the fastest growing fields in chemistry. Studies of molecular recognition,
transport biological aspets, selective catalysis the macrocycle containing the mixed sites of coordination, complexing with Ru (II) gives
catalytic properties of SOD type and catalase [1,2],
inclusion phenomena, organic synthesis and industrial applications of macrocyclic compounds are all burgeoning inmany drection. In this work we
describe the synthesis of new macrocyclic ligand containing pyrazole and aminic coordination sites.
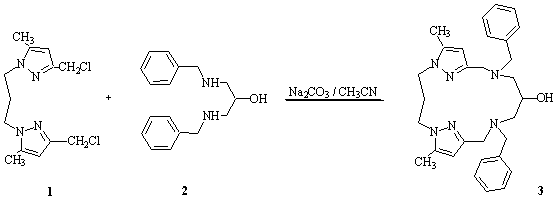
A suspension of sodium carbonate (12 g, 120 mmol) in acetonitrile (250 mL) was refluxed under magnetic stirring, then a solution
of 1,3-bis(3-chloromethyl-5-methylpyrazolyl)propane 1 (2.1 g, 7 mmol) [1] and 1,3-Bis-benzylamino-propan-2-ol 2 (1.9 g , 7 mmol) [3] in acetonitrile (50 mL) was added dropwise. The
solution was refluxed under stirring for two hours, filtered and the solvent
was removed in vacuum, the residue was purified on alumina column with (CH2Cl2/MeOH:
95/5) as eluent to give the 2.62 g (75% yield) macrocycle 3 as an oily substance.
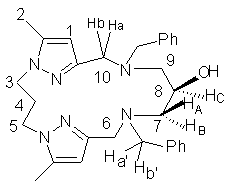
1H-NMR (250 MHz; CDCl3): ¦Ä= 7.3-7,8
(m, H Ph); 5.85 (e, 2H, H1); 4.06 (t, 4H, H3,5); 3.96 (m,
1H, HC JBC
=3,33 Hz JAC =4,99 Hz);
3.77 (d, 2H, Ha J= 9,09
Hz); 3.67 (d, 2H, Hb J= 9,09 Hz); 3.58 (d, 1H, Ha¡¯,
J = 9,08 Hz); 3.48 (d, 1H, Hb¡¯,
J = 9,08 Hz); 2.58 (q, 2H, HB JAB= 8,33 Hz JBC
=3,33 Hz); 2.49 (q, 2H, HA
JAB = 8,33 Hz JAC
=4,99 Hz); 2.34 (t, 2H, H4);
2.20 (d, 6H, H2).
MS
(FAB; m/z): 499 [M+H]+.
References:
1. Tarrago, G.; El Kadiri, S.; Marzin, C. and Coquelet, C. New. J. Chem., 1991, 15, 677.
2. (a) Bienvenue, E.; Choua, S.; Lobo-Recio,
M-HAS.?????; Marzin, C.; Pacheco, P.; Seta, P. and Tarrago, G. J. Inorg. Biochem., 1995, 57, 157.
3. Medou, M.; Priem,
G.; Quelever, G.; Camplo,
M. and Kraus, J. L. Tetrahedron Letters, 1998, 36, 4021
Sample Availability: Available from MDPI.
© 2005 MDPI. All rights
reserved.