|
Molbank 2005, M451 |
Synthesis of New bis-Bidentate Nitrogen
Ligand: 1,4-bis[(3,5-dimethyl-1H-pyrazol-1-yl)methyl]piperazine
Abdeljalil EL FATMI,a Maria DAOUDI,a,* Najib
BEN LARBI,a Abdelali
KERBAL,a Mohamed FILALI BABA,b Brahim EL
BALI,b Michael BOLTE,c
Mohamed MCHICH,d Taibi
BEN-HADDA d,*
a Laboratoire de Chimie Organique, D¨¦partement de Chimie, Facult¨¦ des Sciences Dhar El Mehraz,
b Laboratoire d'Analyses, d'Essais et d'Environnement (LAEE), D¨¦partement de Chimie, Facult¨¦ des Sciences Dhar Mehraz, Universit¨¦ Sidi Mohamed Ben Abdellah, 30000 F¨¨s, Morocco
c Institut fur Organische Chemie, J. W.
Goethe-Universitat Frankfurt, Marie-Curie-Str. 11, 60439 Frankfurt/Main, Germany
d Laboratoire d¡¯activation mol¨¦culaire, Department of Chemistry, Faculty of Sciences,
60000
e-mail: [email protected] and [email protected]
Received:
Keywords: bis-Bidentate
N Ligand - Pyrazole ¨C Amonium/ alcohol condensation.
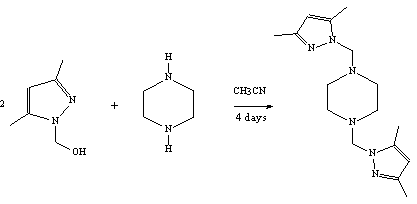
The product
2 was prepared by the addition of piperazine
(C4H10N2) to 1 [1] according to the
reported procedure [2]. To a solution of the substituted hydroxymethylpyrazole 1
(
Melting point: 158-160¡ãC.
IR (KBr, cm¨C
1): 2270 (CH); 1650 (C=C, C=N).
1H- NMR (60 MHz, CDCl3): ¦Ä= 5.8 (s, 2H, Pyrazol,
H4,4'); 4.6 (s, 4H, 2NCH2N);
2.6 (s, 8H, 4CH2-N); 2.30 (s, 12H, 4CH3).
13C-NMR (300MHz, D2O):
¦Ä= 9.64 (Pz-CH3); 12.12 (Pz-CH3);
69.4 (Pz-CH2-N); 81.75 (N-CH2_CH2_N);
106.38(PzC-H); 141.66 (PzC=N);
149.94(PzC-N).
EI-MS (m/z; %): Calculated for C16H26N6:
302.418. Found: 303[M+1]; 109; 95.
Acknowledgments
We are indebted to the "Projet
Global d¡¯Universit¨¦ Mohamed Premier" for
financial support.
References
1. Dvoretzky,
I.; Richter, G.H., J.Org.Chem., 1950, 15, 1285.
2. Sheu, S-C; Tien, M-J.; Cheng,
M-C.; Ho, T-I.; Peng, S-M. And Lin, Y-C., J. Chem.
Soc.
Sample
Availability:
Available from the authors and MDPI.
© 2005 MDPI. All rights
reserved.