|
Molbank 2006, M478 |
Microwave assisted esterification
using Fe2(SO4)3.4H2O/concentrated
H2SO4 as efficient catalyst
Krunal G. Desai1,*, Kishor R. Desai1 and D. Padmanabhan2
1Department of Chemistry, Synthetic Organic Chemistry
Research Laboratory,
Tel: (0261) 2258384, Fax: (0261) 2258384
2Board of Radiation and Isotope Technology, Vashi Complex, Navi Mumbai-400
705 (Maharastra),
e-mail: [email protected]
*Author to whom correspondence should be addressed
Received: 27 June 2005 / Accepted: 6 September
2005 / Published: 31 March 2006
Keywords: esterification, Fe2(SO4)3.4H2O/con.H2SO4
as catalyst, microwave effect
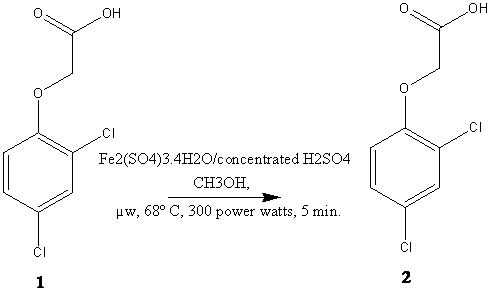
2, 4-D 1 (0.221
g, 1 m mole), Fe2(SO4)3.4H2O
(0.423 g, 1 m mole) and conc. H2SO4
(0.098 mL) in absolute methanol (20 mL) was taken in RBF placed in a microwave oven and
irradiated (300w, 67-68oC) for 5 min [1]. Upon completion of
reaction (monitored by TLC), using petroleumether-ethylacetate
(8:2) as the eluent solvent system. The reaction mixtures
was allowed to attain room temperature, after the completion of reaction
the solvent was removed by vacuum distillation and
treated with cold water. The liquid product separated washed with water to
furnish compound 2, yield 90%.
Melting point: 134-136 oC
IR (KBr) (cm-1): 1722 (>C=O of ester), 1225, 1044 (C-O-C), 3023 (C-H, aromatic ring), 1510 (C=C, aromatic ring), 825 (Ar-Cl).
1H-NMR (CDCl3-DMSO-d6) (400 MHz): ¦Ä= 6.70-7.90 (3H, m, Ar-H), 4.0 (3H, s, -COOCH3), 4.46 (2H, s, -CH2).
13C-NMR (CDCl3-DMSO-d6) (62.90 MHz): ¦Ä= 115.29-134.1 (aromatic carbons), 170 (>C=O of ester), 20 (-COOCH3), 35 (-CH2).
MS (m/z): 235 (M+) (C9H8O3Cl2+), 204 (C8H5O2Cl2+), 176 (C7H5OCl2+), 59 (C2H3O2+), 31 (CH3O+).
Elemental
Analysis: Calculated for C9H8O3Cl2:
C 45.95, H 3.40; found: C 45.98, H 3.43.
Acknowledgment
The authors thank the Veer Narmad South Gujarat University Surat Lucknow
References
1. Desai, K. G.; Desai, K. R. Indian J. Chem. (Sec-B). 2005, (In Press).
Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.