|
Molbank 2006, M501 |
1-Nitro-3-[(benzylsulfonyl)methyl]benzene
Daniel G. Grohmann and Bruce A. Hathaway*
Department of Chemistry, MS
6400, One University Plaza, Southeast Missouri State University, Cape
Girardeau, MO, USA,
Tel. 001 573-651-2370, e-mail: [email protected]
*Author to whom correspondence
should be addressed
Received: 6 July 2006 / Accepted: 1 August 2006 /
Published: 1 September 2006
Keywords: sulfide, sulfone,
oxidation, hydrogen peroxide.
In the course of our work
to prepare inhibitors of the enzyme dihydrofolate reductase, we desired to prepare sulfone
analogues of some similar sulfides [1, 2]. Therefore, we prepared the nitrosulfide 1,
and oxidized it with hydrogen peroxide in acetic acid [3, 4] to prepare the
corresponding nitrosulfone, 2.
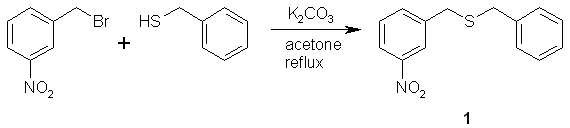
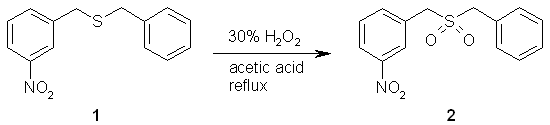
A mixture of 3-nitrobenzyl bromide (6.482 g, 30.00 mmoles), benzyl mercaptan (3.744 g, 30.14 mmoles), and potassium carbonate (4.247 g, 30.73 mmoles) were added to a round bottom flask containing 60 mL acetone. The reaction mixture was refluxed overnight. The potassium bromide that formed in the reaction was removed by vacuum filtration. The acetone was removed from the filtrate by using the rotavap to yield the crude 1-nitro-3-(benzylthio)methyl]benzene, 1, as a dark viscous liquid in quantitative yield. This product was used without further purification in the next reaction.
A mixture of 1-nitro-3-[(benzylthio)methyl]benzene, 1, (5.192 g, 20.02 mmoles), 10 mL of 30% hydrogen peroxide, and 40 mL glacial acetic acid were combined in a round bottom flask and refluxed for 24 hours. The reaction mixture was allowed to cool. The solid which had formed was collected via vacuum filtration and allowed to dry, to yield 4.336 g (14.88 mmoles) of 1-nitro-3-[(benzylsulfonyl)methyl]benzene, 2. The percent yield of this reaction was 74%.
Melting Point: 151 oC
(decomposes)
IR (cm-1): 1520, 1351, 1319, 1297, 1280,
1112, 809, 730, 712, 695, 680, 671.
1H-NMR (300 MHz,
DMSO-d6): ¦Ä= 8.25 (1H,
doublet, J = 8 Hz), 8.15 ppm (1H, singlet), 7.7 (1H,
doublet, J= 8 Hz), 7.55 (1H, triplet, J = 8 Hz), 7.4 (5H, multiplet),
4.3 (2H, singlet), 4.2 (2H, singlet).
13C-NMR (75 MHz, DMSO-d6): ¦Ä= 148.3, 137.1, 130.7, 129.9, 129.4, 129.3, 129.2, 127.2, 125.9, 124.0, 59.4,
56.7.
GC-MS [E.I., m/z (relative intensity)]: 91 (100), 89
(10), 77 (10), 291 (M+, trace).
Acknowledgment
The authors thank the
Grants and Research Funding Committee of Southeast Missouri State University
for financial support.
References:
1. Selassie, C.D., Guo, Z.-r., Hansch, C., Khwaja, T. A., and Pentacost, S. A comparison of the Inhibition of Growth of Methotrexate-resistant and ¨CSensitive Leukemia Cells in Culture by Triazines. Evidence of a New Mechanism of Cell resistance to Methotrexate. J. Med. Chem. 1982, 25. 157-161.
2. Hansch, C., Hathaway, B.A., Guo, Z.-r., Selassie, C.D., Dietrich, S.W., Blaney, J.M., Langridge, R., Volz, K.W., and Kaufman, B.T. Crystallography, QSAR, and Molecular Graphics in a Comparative Analysis of the Inhibition of Dihydrofolate Reductase from Chicken Liver and L. casei by 4,6-Diamino-1,2-dihydro-2,2-dimethyl-1-(x-phenyl)-s-triazines. J. Med. Chem. 1984, 27, 129-143.
3. Rheinboldt, H.; Giesbrecht, E. The Configuration of Sulfoxides.
Mixed
4. Ongley, P.S., Ed. Organicum: Practical Handbook of Organic
Chemistry. Addison-Wesley Publishing Company, Inc,
© 2006 MDPI. All rights reserved.