| Molbank 2007, M525 |
Synthesis
of N-(N¡¯-benzoylhydrazinomethyl)benzamide
Institute of Chemistry, Faculty of Natural Sciences & Mathematics, Sts. Cyril and Methodius University, Arhimedova 5, PO Box 162, 1000 Skopje, Macedonia
Tel: +389 (0)2
3249918; Fax: +389 (0)2 3226865; E-mail:
[email protected]
Received: 9
January 2007 / Accepted: 21 January 2007 / Published: 31 May 2007
Keywords:
Benzamidomethylation, aqueous
media, hydrazide
Our previous
research has shown that (benzamidomethyl)triethylammonium
chloride (1) is an excellent benzamidomethylating
agent for different types of compounds in aqueous media because of the
mild
reaction conditions, high yields and simple isolation of products
[1-3].
In the course
of this work, we have also carried out reaction of 1
with benzhydrazide (2)
in
aqueous media.
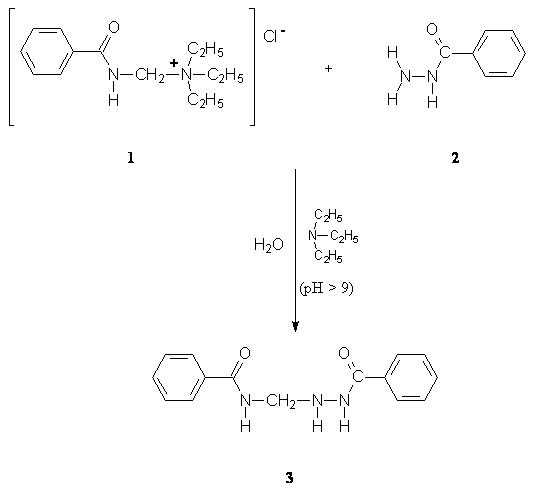
A solution of (benzamidomethyl)triethylammonium
chloride (1) (0.273 g; 1.01 mmol) in water (10 mL) was added
in small portions to a solution of benzhydrazide
(2)
(0.204 g; 1.50 mmol) in
water (20 mL).
In the same time, triethylamine
(TEA) (0,2-0,4 mL; pH >9), drop by drop
was added to the mixture. The
mixture was stired for
4-5 h at room temperature,
afterward, 30 min. in ice bath.
Colorless crystals were collected by filtration and
purification was
performed by recrystallization
from dioxane.
Yield: 86 %
Melting Point:
144 oC
(dioxane)
IR (KBr; cm-1):
¦Í(N-H) 3329
and 3302; Amide I 1651 and 1638; Amide II 1543.
1H-NMR (DMSO-d6;
250 MHz); ¦Ä/ppm = 10.03
(d, 1H, N-NHCO); 8.85 (t, 1H,
CONH-C); 7.83-7.40 (m, 10H, Ar);
5.67 (q, 1H, C-NH-N)
and 4.34 (t, 2H, CH2).
13C-NMR (DMSO-d6;
63 MHz); ¦Ä/ppm = 167.0
C=O; 165.6 C=O; 56.3 CH2,
Ar: 134.6; 133.4; 131.3;
128.3; 127.3 and 127.2.
Elemental
Analysis: Calculated (%) for C15H15N3O2:
C 66.9, H 5.6, N 15.6.
Found: C 66.7, H 5.7, N
15.3.
References and Notes:
1.
Popovski, E.; Klisarova, L.; Vikic-Topic, D.
Simple Method for Benzamidomethylation
of Phenols in
Water Solution. Synth. Commun.
1999, 29,
3451-3458.
2.
Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (Benzamidomethyl)-triethylammonium Chloride. 2. A
Simple Method for Benzamidomethylation
of Thiols,
Amines and Carboxylic acids. Molecules 2000, 5,
927-936.
3.
Popovski, E.; Bogdanov, J.; Najdoski, M. and
Hey-Hawkins, E. Reactions of (Benzamidomethyl)triethylammonium
Chloride with
Some Inorganic Nucleophiles
in Aqueous Media. Molecules 2006, 11,
279-285.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.