|
Molbank 2007, M529 |
Synthesis of Ethyl
(4-benzyl-3-methyl-6-oxopyridazin-1(6H)-yl)acetate
Nour-Eddine Benchat *, Abderrahmane
Anaflous, Said Abouricha, Mohammed
Ramdani and Abderazak Benalla
*Author to whom correspondence should be addressed. E-mail: [email protected]
Received: 3 January 2006 / Accepted: 24 June 2006 / Published: 31 May 2007
Keywords: Pyridazines, Anticancer activity
Wermuth and coll
[1] synthesized a series of products by alkylation of
pyridazines, Laborit [2]
showed that these products are good analgesics and have a low toxicity. In continuation of this line of
investigation, we have synthesized compound (I); it will be subjected to further pharmacological
investigations, especially tests of its anticancer activity.
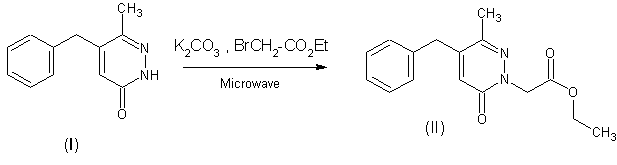
The product
(II) was prepared from
5-benzyl-6-methylpyridazin-3(2H)-one (I)
in situ by the solid-liquid PTC conditions without solvent[3].
To pyridazin (I)
(
Melting point: 89-
IR (KBr, cm-1): 1740 (CO2Et), 1603, 1469, 1211 (C = N).
1H-NMR (300.14 MHz, CDCl3) d (ppm): 1.29 (t, J=5Hz, 3H, CH3), 2.23 (s, 3H, CH3), 3.78 (s, 2H, CH2), 4.26 (q, J = 5Hz, 2H, CH2), 6.53 (s, 1H, H4), 7.12 (d, J = 7.5Hz, 2H, aromatic protons), 7.25 (m, 3H, aromatic protons).
13C-NMR (75 MHz, CDCl3) d (ppm):19.12(CH3),
38.45 (CH2), 52.67 (CH2), 61.65 (CH2), 127.26
(CH aromatic), 127.97 (CH aromatic), 129.02 (2 CH aromatic), 129.11 (2 CH
aromatic), 135.61, 145.21, 146.42, 160.38 (C3), 167.74 (C=O).
MS: m/z (%): M+= 287,
241.
References:
1. Wermuth, C.G.; Leclerc G.; Melounov, P. Chim. Ther. 1971, 2, 109.
2. Laborit, H.; Weber, B.; Wermuth,
C.G.; Delbarre, B.; Chekler,
C.; Baron, C.; Rosen Garten, H. AGRESSOLOGIE 1965, 6, 415.
3. (a) Kappe, C.O.; Dallinger, D. Nature Reviews Drug Discovery 2006, 5, 51. (b) De
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.