|
Molbank 2007, M533 |
Synthesis of N-acetyl-N-(3,5-dioxo-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-4-yl)-acetamide
Marta Struga 1*,
Jerzy Kossakowki 1,
Barbara Mirosław 2, Anna E. Kozioł 2
1 The Medical University,
Department of Medical Chemistry, 3 Oczki Str., 02-007 Warsaw, Poland; Tel/Fax: (+4822)-628-06-79
2 Faculty of Chemistry,
* Author to whom correspondence should be addressed. E-mail: [email protected]
Received:
27 September 2006 / Accepted: 16 January 2007 / Published: 31 May 2007
Keywords: 4-amino-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione, acylation
Various imide derivatives of 10-Oxa-4-aza-tricyclo[5.2.1.02,6]decane-3,5-dione have been reported and shown to exhibit a wide spectrum of biological activities including antitumor properties [1].

4-Amino-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione (1) was used as a starting material. This compound was obtained in Diels-Alder reaction of furan and furan-2,5-dione [2] and next treated with hydrazine (80% aqueous solution) [3]. Compound 2 was obtained in acylation reaction of compound 1. The reduction of compound 2 occurred during the acylation.
N-acetyl-N-(3,5-dioxo-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-4-yl)-acetamide
(2).
0.01 Mole of the compound 1 and 10 ml of acetic anhydride were heated while boiling for 6h under reflux condenser. The reaction mixture was filtered off and the solvent was removed under a reduced pressure. The residue was crystallized from ethanol. Next it was purified by column chromatography (silica gel) using chloroform/methanol (19:1) as eluent.
White crystals, yield 78 %.
Melting point: 128 ºC.
1H NMR (400 MHz, CDCl3) ¦Ä (ppm): 4.96 (s, 2H, CH-O);
3.1 (s, 2H, CH-C=O); 2.59 (s, 3H, CH3); 2.11 (s, 3H, CH3);
1.93 (m, 2H, CH2); 1.68 (m, 2H, CH2).
13C-NMR (100 MHz, CDCl3) ¦Ä (ppm): 174.3, 136.1, 79.8, 45.0, 38.8.
ESI MS: m/z = 289.2 [M + Na]+
(100%).
Elemental Analysis: Calculated for C12H14N2O5 (266.25) calculated: C, 54.13 %; H, 5.30%; N, 10.52 %. Found: C, 54.18 %; H, 45.32 %; N, 10.72 %.
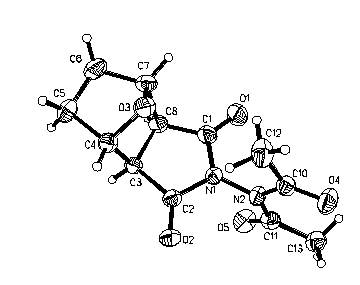
Cis, exo configuration at the ring junction; the N,N-diacetyl fragment is planar with perpendicular orientation to the imid ring plane; the C=O bonds of acetyl groups are anti.
The
diffraction data were collected at 275 K on a KM-4 diffractomater
using the crystal of dimensions 0.22 ´
0.15 ´ 0.11 mm and CuKa radiation. Within the q
range of 5.3 to 72.2¡ã, 2445 reflections
were collected. The structure was solved by direct methods and refined by
full-matrix least-squares on F2
(programs SHELXS97 and SHELXL97 [4, 5]). The refinement of 175 parameters
converged at final R indices:
R1
= 0.0311, wR2 = 0.0889 (for
1039 observed reflections, I > 2s
(I)) and R1 = 0.1377, wR2
= 0.1188 (all data), and Goof = 0.996. The extinction coefficient was
0.0032(3), residual electron density Dr
(max) = 0.20 and Dr
(min) = -0.18 e A-3.
|
N(1)-N(2) |
1.383(2) |
C(3)-C(4) |
1.525(3) |
|
N(1)-C(1) |
1.393(3) |
C(3)-C(8) |
1.542(3) |
|
N(1)-C(2) |
1.396(3) |
C(4)-C(5) |
1.529(3) |
|
N(2)-C(10) |
1.416(2) |
C(5)-C(6) |
1.537(3) |
|
N(2)-C(11) |
1.420(3) |
C(6)-C(7) |
1.521(3) |
|
O(1)-C(1) |
1.205(3) |
C(7)-C(8) |
1.542(3) |
|
O(2)-C(2) |
1.201(2) |
C(10)-C(12) |
1.490(3) |
|
O(3)-C(4) |
1.440(2) |
C(11)-C(13) |
1.486(3) |
|
O(3)-C(7) |
1.442(3) |
N(2)-N(1)-C(1) |
122.5(2) |
|
O(4)-C(10) |
1.198(2) |
N(2)-N(1)-C(2) |
123.2(2) |
|
O(5)-C(11) |
1.199(2) |
C(1)-N(1)-C(2) |
114.0(2) |
|
C(1)-C(8) |
1.489(3) |
C(1)-N(1)-N(2)-C(10) |
93.2(2) |
|
C(2)-C(3) |
1.508(2) |
C(2)-N(1)-N(2)-C(11) |
90.6(2) |
Table 1.
Bond lengths (Å)
References:
1. Walter, W.G. J. Pharm. Sci. 1989, 78, 66.
2. Kwart, H.; Burchuk, J. J. Am. Chem. Soc.
1952, 74, 3094.
3. Struga, M.; Mirosław, B.; Wawrzyca-Gorczyca, I.; Kossakowski,
J.; Kozioł, A.E. Polish J. Chem. 2007, 81, 51.
4. G.M. Sheldrick, SHELXS-93: Program for crystal structure solution;
5. G.M. Sheldrick,
SHELXL-97: Program for the refinement of crystal structures from diffraction
data;
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.