|
Molbank 2007, M534 |
Synthesis of 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione
Marta Struga * and Jerzy Kossakowski
The
Medical University, Department of Medical Chemistry, 3 Oczki
Str., 02-007 Warsaw, Poland; Tel/Fax: (+4822)-628-06-79
*Author
to whom correspondence should be addressed. E-Mail: [email protected]
Received:
27September 2006 / Accepted: 16 January 2007 / Published: 31 May 2007
Keywords: 1,7,8,9-Tetramethyl-4-oxa-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione, 4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione, hydrazine hydrate
Various imide derivatives of 1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione (2) have been reported and shown to exhibit an anxiolytic activities [1].
Starting materials e.g. 1,2,3,4-tetramethyl-1,3-cyclopentadiene, and furan-2,5-dione were commercially available reagents.
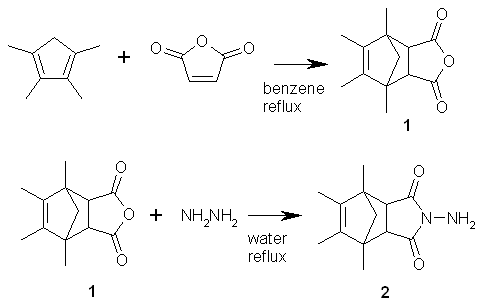
1,7,8,9-Tetramethyl-4-oxa-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione (1).
The synthesis of (1) was performed from 1,2,3,4-tetramethyl-1,3-cyclopentadiene and furan-2,5-dione according to the method described previously [2]. (Lit. m.p. 108-1090C. Yield 68 %.)
White crystals, yield 76 %.
Melting point: 110ºC.
1H NMR (400 MHz, CDCl3) ¦Ä (ppm): 3.35 (s, 2H, CH-C=O); 3.29
(s, 12H, CH3); 2.72 (s, 2H, CH2).
13C-NMR (100 MHz, CDCl3) ¦Ä
(ppm): 171.3, 138.4, 137.6, 63.9, 56.3,
54.4, 16.6, 11.0.
ESI MS: m/z
= 243.3 [M + Na]+ (100%).
Elemental Analysis: Calculated for C13H16O3 (220.26): C, 70.89 %; H,
7.32 %. Found: C, 70.92 %; H, 7.23 %.
4-Amino-1,7,8,9-tetramethyl-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione
(2).
A mixture of anhydride 1 (0.01 mole) and hydrazine hydrate (80% water solution) (10 ml) was refluxed for 5 h. The solvents were evaporated. The residue was purified by a column chromatography (silica gel, chloroform/methanol 19:1) to give compound 2.
White crystals, yield 89 %.
Melting point: 161ºC.
1H NMR (400 MHz, CDCl3) ¦Ä (ppm): 4.52 (s, 2H, NH2);
3.02 (s, 2H, CH-C=O); 1.53 (s, 6H, CH3); 1.47 (s, 6H, CH3);
1.33 (dd, 2H, CH2).
13C-NMR (100 MHz, CDCl3) ¦Ä
(ppm): 174.4, 137.6, 63.6, 55.2,
51.9, 17.1, 10.9.
ESI MS: m/z
= 257.2 [M + Na]+ (100%).
Elemental
Analysis: Calculated for C13H18O2N2 (234.3): C, 66.07 %; H, 8.53 %;
N, 11.85 %. Found: C, 66.32 %; H, 8.32 %; N, 11.89 %.
References:
1. Kossakowski, J.; Kuśmierczyk, J. Acta Pol. Pharm. 1999, 56, 94.
2. Mironov, V.A.; Sobolev, E.V.; Elizarova, A.N. Tetrahedron 1963, 19, 1939.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.