|
Molbank 2007, M536 |
2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline
Caleb Ahoya Anothane,
Rachid Bouhfid and El Mokhtar Essassi *
Laboratoire de Chimie
Organique H®¶t®¶rocyclique, Pôle de comp®¶tences Pharmacochimie, Universit®¶
Mohammed V-Agdal, BP: 1014 Avenue Ibn
Batouta,
*Author to whom correspondence
should be addressed. E-mail: [email protected]
Received: 9 November 2006 / Accepted: 4 December 2006 /
Published: 31 May 2007
Keywords: quinoxaline, thiophene, fusion.
Heterocyclic compounds
particularly five and six membered ring compounds have occupied a
prominent place among various classes of organic compounds for their
diverse biological activities. Among a wide variety of heterocycles
that have been explored for developing pharmaceutically important
molecules. Quinoxaline and thiophene have played an important role in
medicinal chemistry. Some of them have received considerable attention
as potential antimicrobial1 [2], and antiviral [3,4].
The aim of this work is to describe the synthesis of a novel compound entitled 2-(3-thienyl)-2,3-dihydrofuro[2,3-b]quinoxaline.
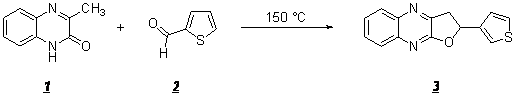
The 3-methylquinoxalin-2(1H)-one 1 (1.25 mmol, 2 g) and 3-formyl-thiophene 2 (3.75 mmol, 4.8 mL) were heated in a bath oil at 150 °„C for 3h. After cooling of the reaction, the crude product was recrystallized from ethanol to obtain compound 3.
Melting point: >
¹H-NMR (300 MHz, DMSO-d6): 3.17, 3.20 (m, 2H, HaHb, JAB = 14.7 Hz, JAX = JBX = 7.2 Hz); 4.28 (q, 1H, Hx, JAX = JBX = 7.2 Hz); 7.05-7.62 (m, 7H, HAr).
¹³C-NMR (300 MHz, DMSO-d6): 35.5 (CH); 48.5 (CH2); 115.4, 120.6, 123.3, 126.0, 127.8, 128.3, 129.7 (CHAr); 131.9, 132.0, 146.4, 155.1, 160.8 (Cq).
MS (EI): M+(m/z = 254, 46%); 143 (100%).
Elemental analysis: Calculated for C14H10N2OS: C, 66.12 %; H, 3.96 %; N, 11.02 %; Found: C, 66.22 %; H, 4.01 %; N, 11.12 %;
References:
1. Carta, A.; Paglietti, G.; Nikookar, M.E.R.; Sanna, P.; Sechi, L.; Zanetti, S. Eur. J. Med. Chem. 2002, 37, 355.
3. Fonseca, T.; Gigante, B.; Marques, M.M.; Gilchrist, T.L.; De Clercq, E. Bioorg. Med. Chem. 2004, 12, 103.
4. Sehlstedt, U.; Aich, P.; Bergman, J.; Vallberg, H.; Nord®¶n, B.; Gräslund, A. J. Mol. Bio. 1998, 278, 31.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.