2-[5-chloro(bromo)-3-hydrazono-2-oxo-2,3-dihydro-1H-indolin-1-yl]acetohydrazide
Bouhfid Rachid 1, Nicolas Joly 2, Vincent Lequart 2, Patrick Martin 2, Mohammad Massoui 1 and El Mokhtar Essassi 1,*
1 Laboratoire de Chimie Organique H®¶t®¶rocyclique, Pôle de comp®¶tences Pharmacochimie Universit®¶ Mohammed V-Agdal, BP:
1014 Avenue Ibn Batouta, Rabat, Morocco
2 Blood-Brain Barrier Laboratory (EA 2465), IUT of B®¶thune,
University of Artois, BP 819, F-62408 B®¶thune
*Author to whom correspondence
should be addressed. E-mail: [email protected]
Received: 9 November 2006 / Accepted: 4 December 2006 /
Published: 31 May 2007
Keywords: isatin, hydrazone, indoline, acetohydrazide.
Isatin derivatives exhibit significant biological,
medicinal and pharmacological activities, [1,2] such as
antituberculous, antihypoxing agent, anticonvulsant, antihyperglycemic; active
against salmonella, typhi and against vibrio cholerae.
Besides, they are used in treating and preventing pestvirus. [3]
We describe in this work, the synthesis of new
indoline derivatives.
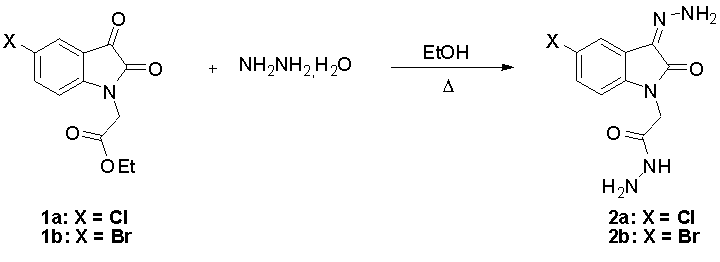
To a solution of 1a-b
(3.7 mmol) in 10 mL of ethanol, was added hydrazine hydrate (7.4 mmol). The
mixture was refluxed for 6 h. After cooling the precipate was filtered and
recrystallised from ethanol to afford product 2a-b.
2-[5-chloro-3-hydrazono-2-oxo-2,3-dihydro-1H-indolin-1-yl]acetohydrazide 2a:
Yield: (74%). Melting Point: > 250°„C. MS (IE): M+( m/z) = 267 (76%)/ 269 (25%);
180 (100%). ¶‘ (cm-1): 3310, 3285, 3113, 3091, 1675, 1654, 1598,
1532. 1H NMR (300 MHz, CDCl3): 3.52 (s, 2H, NH2),
4.12 (s, 2H, NH2); 4.61 (s, 2H, NCH2); 7.00-7.40 (m, 3H,
HAr); 9.27 (s, 1H, NH). 13C NMR (300 MHz, CDCl3):
41.2 (NCH2);
111.2, 117.6, 126.9 (CHAr); 117.8, 124.6, 138.4 (Cq); 159.1
(C=Oamide); 161.4 (C=Nimine); 165.3 (C=Ohydrazide). Elemental analysis: Calculated for C10H10ClN5O2:
C, 44.87%; H, 3.77%; N, 26.16%; Found: C, 44.62%; H, 3.88%; N, 25.96%.
2-[5-bromo-3-hydrazono-2-oxo-2,3-dihydro-1H-indolin-1-yl]acetohydrazide 2b:
Yield: (70%). Melting
Point: > 250°„C. MS(IE): M+(m/z) = 311 (39%)/ 313 (37%); 224 (100%). ¶‘ (cm-1): 3320, 3279, 3162,
3089, 1687, 1656, 1599, 1533. 1H NMR (300 MHz, CDCl3): 3.34
(s, 2H, NH2), 4.06 (s, 2H, NH2); 4.57 (s, 2H, NCH2);
6.91-7.51 (m, 3H, HAr); 9.32 (s, 1H, NH). 13C NMR (300 MHz,
CDCl3): 40.6 (NCH2); 111.4, 120.4, 129.6 (CHAr);
115.0, 124.6, 138.7 (Cq); 159.0 (C=Oamide); 161.2 (C=Nimine);
167.2 (C=Ohydrazide). Elemental analysis: Calculated for C10H10BrN5O2:
C, 38.48%; H, 3.23%; N, 22.44%; Found: C, 38.31%; H, 3.35%; N, 22.56%.
References:
1. Pandeya, S.N.; Sriram, D. Acta
Pharm. Turc. 1998, 40,
33.
2. Sarangapani, M.; Narayan, A.R.; Jayamma, Y.; Reddy, V.M. Indian Drugs 1998, 35, 336.
3. Pevear, C.D.; Nitz, J.T.; Seipel, M. PCT Int. Appl. WO98
36,752. (Cl.A61K3 1/555)27 Aug
1998, US Appl. 12 Feb. 1997, 803, 675.
4. Joshi, K.C.; Jam, R.; Chand, P.; Garg, S. J. Indian Chem. Soc.
1983, LX, 760.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
