|
Molbank 2007, M543 |
One-pot Synthesis of
2-seleno-4-methylquinoline
Tangali R. Ravikumar Naik,
Halehatty S. Bhojya Naik *, S. Ramesh, M. Raghavendra and G.
Krishnamurthy
Department of Studies and Research in Industrial Chemistry, School of
Chemical Sciences,
* Author to whom correspondence should be addressed. Fax: +91-08282-256255; E-mail: [email protected]
Received: 17 December 2006 / Accepted:
3 January 2007 / Published: 2 June 2007
Keywords: Quinoline, DNA binding, antitumour, organoselenium
Number
of workers has reported the synthesis of condensed fused quinoline
derivatives containing selenium atom and studied for their DNA binding, cytotoxic, anticancer and antitumour
activities [1-3]. The reported ring system of interest
because of close relationship with anticancer
alkaloid ellipticine [4]. Therefore, the
development of organoselenium compounds with higher anticarcinogenic efficacy but better tolerance continues to
be a priority in chemotherapy research.
So, we have tentatively identified these 2-selenium-4-methylquinolines
as new member of the class of antitumour drug and
starting material for the synthesis of many tri- and tetra cyclic
planar molecules. We herein report facile, eco-friendly and one-pot synthesis
of 2-seleno-4-methylquinoline from 2-chloro-4-methylquinoline.
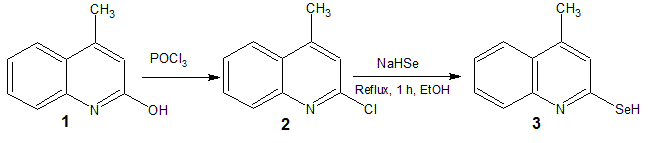
The synthesis of 2-hydroxy-4-methylquinoline and 2-chloro-4-methylquinoline were prepared according to literature procedure [5].
A mixture of 2-chloro-4-methylquinoline 2 (1.58 g, 1 mmol) and NaHSe (1 g, 1 mmol) was refluxed at 80-90 oC in presence of ethanol (5 ml) for 1 h. The completion of the reaction was monitored by TLC eluting the phase ethyl acetate: carbon tetrachloride (80:20). The reaction mixture was poured in to crushed ice (25 gm). The product was filtered, washed with water, dried and was pure enough for further use. The obtained compound was characterised by elemental analysis, IR, 1H NMR, 13C NMR and mass spectral data. The 2-seleno-4-methylquinoline 3 has a yellow, solid powder, yield 80 %.
Melting Point: 170-173 oC
IR (KBr, cm-1): n 1558 (C=N); n 1634(C-SeH).
1H-NMR (CDCl3, 250 MHz): d = 2.63 (3H, s, CH3), 6.38-7.28 (5H, m, Ar), 10.8 (1H, s, SeH).
13C-NMR (CDCl3, 67.5 MHz): d = 126.0, 127.4, 127.9, 128.0, 130.4, 140.0, 142.2, 143.0, 149.5, 153.5.
Mass Spectra (relative intensity): m/z (M.+2) = 222
Elemental Analysis: Calculated for C10H9NSe: C: 54.07, H: 4.08, N: 6.31, Se: 35.54 Found: C: 54.05, H: 4.07, N: 6.29, Se: 35.52.
References
- Gopal, M.; Veeranna, S.; Doddamani, L.S. Spectroscopy Letters 2004, 37, 347.
- Gopal, M.; Shahabuddin, M.S.; Inamdar, S.R. Indian Acad. Sci. (Chem. Sci). 2002, 114, 687.
- Ravikumar Naik, T.R.; Bhojya Naik, H.S.; Raghavendra, M.; Gopalkrishna Naik, S.R. ARKIVOC 2006, 15, 1.
- Frei, E.; Bieler, C.A.; Arlt, V.M.; Wiessler, M.; Stiborova, M. Biochem. Pharmacol. 2002, 64, 289.
- Bhojya Naik, H.S.; Ramesh, S.; Swetha, B.V.; Roopa Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 533.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.