|
Molbank 2007, M548 |
Synthesis of 4-bromo-2-thiomorpholin-4-ylmethyl-1-phenol
Ana Mar¨ªa Vel¨¢zquez 1,
Luis Alberto Torres 1, Ra¨²l Gonz¨¢lez 1, Alvaro Valencia 1, Sandra D¨ªaz-Barriga 1, Italo Menconi 1,
Luisa Mart¨ªnez 1, Alberto Ram¨ªrez 1, Ignacio Mart¨ªnez 1, Br¨ªgida Camacho 1,
Rafael L¨®pez-Castañares 2 and
Enrique Angeles 1,*
2
Facultad de Qu¨ªmica de la UAEM, Universidad
Aut¨®noma del Estado de M¨¦xico
* Author to whom correspondence should be addressed. E-mail: [email protected]
Keywords:
4-bromophenol, thiomorpholine,
IR
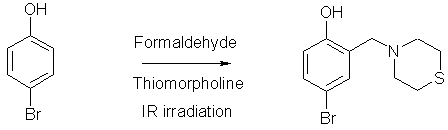
| 1 | 2 |
4-bromo-2-thiomorpholin-4-ylmethyl-1-phenol (2) was prepared from 4-bromophenol (1) and thiomorpholine and formaldehyde (2 eq.) and 1 eq. of thiomorpholine. They were mixed in a round flask fitted with a condenser. The mixture was irradiated with infrared light using a medicinal infrared lamp (250 Watts) and the reaction was monitored by tlc, and after 7 minutes, the reaction was completed. The mixture was chromatographed on silica gel using solvent gradient hexane/ethyl acetate. Yield 63%
Melting point: 130-132 ¡ãC (hexane/ethylacetate, uncorrected).
IR (n cm-1; CHCl3 film): 3520 (O-H), 3010 (Csp2-H Ar), 2985 (Csp3-H).
1H-NMR (200 MHz; CDCl3; Me4Si, ¦ÄH): 10.69 (1H, s, OH), 7.26 ( 1H, dd, J=8.8Hz, J=2.4Hz), 7.082 (1H, d, J=2.4Hz), 6.70 (1H, d, J=8.8Hz), 3.67 (2H, s, Ar-CH2), 2.81 (4H, m, -S-CH2- ), 2.74 (4H, m, -N-CH2-).
13C-NMR (50 MHz; CDCl3; ¦ÄC): 156.7 (C), 131.61 (CH), 131.25 (CH), 122.76 (C), 117.9(CH), 110.88 (C), 61.66 (Ar-CH2), 54.36 (-N-CH2-), 27.84 (-S-CH2-).
FAB-MS m/z (rel%) (M+1): 288(53%), 221, 135
Elemental Analysis: Calculated for C11H14BrONS (287): C 45.99 %, H 4.87 %, N 4.87 %, O 5.57 %, S 11.15 %, Br 25.52, found : C 46.07 %, H 4.98 %, N 4.81 %, O 5.6 %, S 11.23 %, Br 25.34%.
Acknowledgements
The authors wish to acknowledge to
PAPIIT/UNAM Projects No IN213606 and IN207705 and ALPHARMA SA de CV, by
partially support this work. We would like to thank C.Barajas,
F.Sotres, P.Garc¨ªa, D.Jim¨¦nez from FESC-UNAM and Rosa I.del
Villar M., Oscar Yañez and
Georgina Duarte from
USAI-UNAM for their skillful technical assistance and DGSCA-UNAM
for their support. As a part of Project C¨¢tedra
Qu¨ªmica Medicinal of FESC-UNAM.
References
- Vel¨¢zquez, A.Ma.; Torres, L.A.; D¨ªaz, G.; Ram¨ªrez, A.; Hern¨¢ndez, R.; Santill¨¢n, H.; Mart¨ªnez, L.; Mart¨ªnez, I.; D¨ªaz-Barriga, S.; Abrego, V.; Balboa, M.A.; Camacho, B.; L¨®pez-Castañares, R.; Dueñas-Gonz¨¢lez, A.; Cabrera, G.; Angeles, E. ARKIVOC
2006, 2, 150-161.
- Biava, M.; Fioravanti, R.; Porretta, G.C.; Deidda,
D.; Maullu, C.; Pompei, M. Biorg. & Med. Chem. Lett. 1999,
9, 2083-2985.
- Teipel,
S.; Griesar, K.; Haase,
W.; Krebs, B. Inorganic
Chemistry 1994, 33, 456-464.
- Hodgkin, J.H. Aust. J. Chem. 1984,
37, 2371-2378.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.