| Molbank 2007, M556 |
Synthesis of 6-Chloro-N,N,N',N'-tetrakis-pyridin-2-ylmethyl-[1,3,5]triazine-2,4-diamine
Daniel Vomasta, Manfred Zabel and Burkhard König *
Institute of Organic Chemistry, University of Regensburg, D-93040 Regensburg, Germany.
* Author to whom correspondence should be addressed. E-mail: [email protected]
Received: 2 August 2007 / Accepted: 6 September 2007 / Published: 7 September 2007
Keywords: dipicolylamine, 2,4,6-tris-chloro-triazine, phosphate binding
Transition metal complexes of pyridine-containing ligands are widely used in catalysis [1], supramolecular self-assembly [2], and anion recognition [3]. Binuclear Zn2+-2,2’-dipicolylamine (dpa) complexes are particular useful for the binding of phosphorylated peptides in aqueous solution under physiological pH with high affinity and selectivity [4-9]. Similar ligands based on 2,2’-bipyridylamine (bpa) and 2,4,6-tris-chloro-triazine, have been used to form coordination networks with copper(II) ions. In these compounds the bpa moiety is attached directly to the heteroaromatic core. Combination of 2,4,6-tris-chloro-triazine with dpa moieties leads to a new hybrid compound B, which should be used in phosphate binding studies. We describe the versatile short synthesis of the dpa compound B starting from 2,4,6-tris-chloro-triazine A.
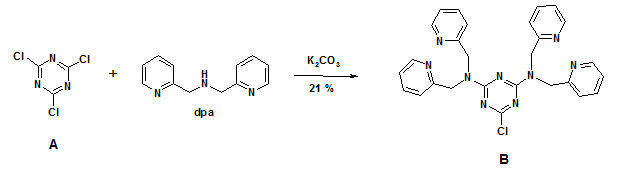
Melting point: > 200°C
1H-NMR (300 MHz, CDCl3): δ = 4.82 (s, 4 H, CH2), 5.03 (s, 4 H, CH2), 6.90 (d, 3J = 8.0 Hz, 2 H), 7.04 (t, 3J = 5.8 Hz, 2 H), 7.16 (t, 3J = 5.6 Hz, 2 H), 7.31 (d, 3J = 8.0 Hz, 2 H), 7.38 (t, 3J = 7.7 Hz, 2 H), 7.64 (t, 3J = 7.7 Hz, 2 H), 8.41 (d, 3J = 4.1 Hz, 2 H), 8.51 (d, 3J = 4.9, 2 H).
13C-NMR (75 MHz, CDCl3): δ = 51.9 (-, 2 C), 52.1 (-, 2 C), 121.3-122.5 (+, 8 C), 136.7 (+, 2 C), 136. 7 (+, 2 C), 149.2 (+, 2 C), 149.3 (+, 2 C), 157.2 (Cquat, 2 C), 157.2 (Cquat, 2 C), 165.7 (Cquat, 2 C), 169.9 (Cquat, 1 C).
ES-MS (DCM/MeOH + 10 mmol/l NH4Ac): m/z (%) = 510.3 (100) [MH+].
Elemental analysis: Calc. C 63.59, H 4.74, N 24.72 found C 63.27, H 4.72, N 24.58; IR (KBr) n (cm-1) = 2927 (w), 2529 (w), 1707 (s), 1571 (s), 1491 (m), 1433 (w), 1410 (w), 1354 (w), 1319 (w), 1237 (w), 1169 (w), 1084 (w), 1048 (w), 972 (w), 947 (w), 889 (w), 863 (w), 804 (w), 756 (m), 683 (w), 617 (w), 554 (w), 458 (w).
Figure 1. 1H-NMR spectrum of compound B
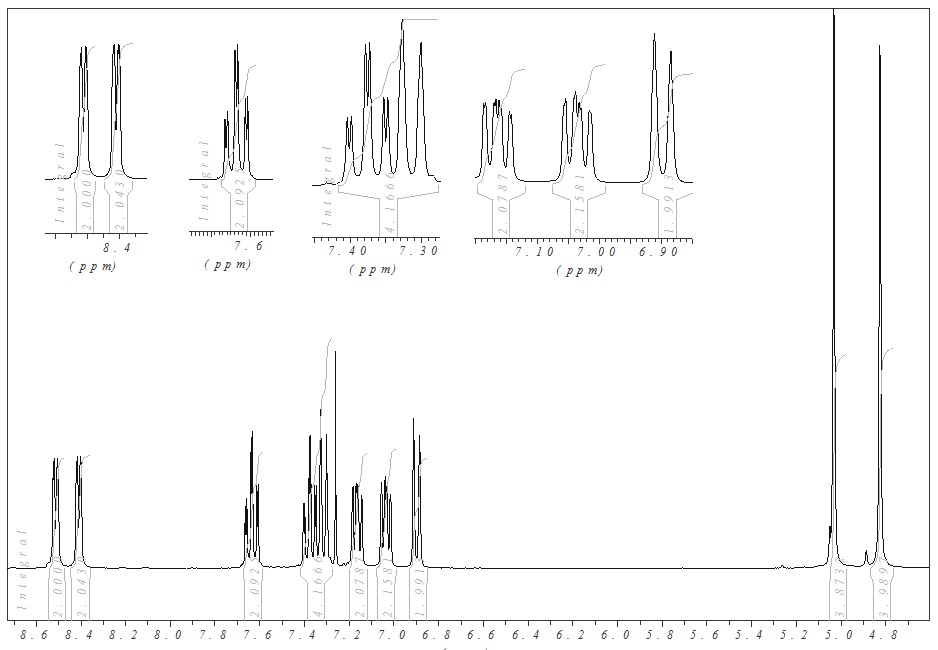
Figure 2. X-ray structure of B. Suitable crystals were obtained by recrystallization from DCM/MeOH 5:1.
Conclusion
A new dpa compound B was prepared in a one step synthesis. The compound can be prepared from commercially available compounds and is accessible by a simple reaction. Surprisingly, in first attempts this compound did not form spontaneously complexes with Zn2+ salts at conditions described for other dpa ligands [4-9].
References
- Gil-Molto, J.; Karlstroem, S.; Najera, C., Tetrahedron 2005, 61, (51), 12168-12176.
- Demeshko, S.; Leibeling, G.; Dechert, S.; Meyer, F., Dalton Trans. 2004, (21), 3782-7.
- Kruppa, M.; Koenig, B., Chem. Rev. 2006, 106, (9), 3520-60.
- Jiang, H.; O'Neil E, J.; Divittorio, K. M.; Smith, B. D., Org. Lett. 2005, 7, (14), 3013-6.
- Ojida, A.; Inoue, M. A.; Mito-Oka, Y.; Hamachi, I., J. Am. Chem. Soc. 2003, 125, (34), 10184-5.
- Ojida, A.; Mito-Oka, Y.; Inoue, M. A.; Hamachi, I., J. Am. Chem. Soc. 2002, 124, (22), 6256-8.
- Ojida, A.; Miyahara, Y.; Kohira, T.; Hamachi, I., Biopolymers 2004, 76, (2), 177-184.
- Ojida, A.; Park, S.-k.; Mito-oka, Y.; Hamachi, I., Tetrahedron Lett. 2002, 43, (35), 6193-6195.
- Yamaguchi, S.; Yoshimura, I.; Kohira, T.; Tamaru, S.; Hamachi, I., J. Am. Chem. Soc. 2005, 127, (33), 11835-41.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.