| Molbank 2008, M564 |
http://www.mdpi.org/molbank/ |
New Route to Metformin Hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) Synthesis
Anvar Shalmashi
Abstract: In this work,
microwave (MW) assisted synthesis of metformin hydrochloride was performed on
thin layer chromatography (TLC) plates. This versatile, simple and economical
green methodology is readily amendable to parallel synthesis of metformin
hydrochloride libraries.
Keywords: metformin hydrochloride, microwave heating, solvent free condition
Introduction
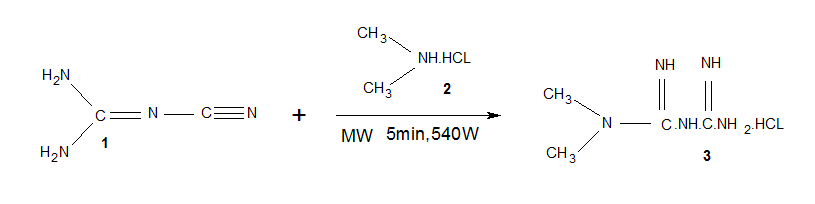
Metformin
hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is
an oral antihyperglycemic drug used in the management of diabetes. It is
usually prepared from the reaction between dimethylamine hydrochloride and
dicyano diamide at 120-140 oC in 4 hrs time with 69% yield [1]. In designing ecofriendly
synthesis of the target molecule, the starting materials are made to react by
modifying the reaction conditions in such a way that the by-products and wastes
are eliminated and also the use of organic solvents is minimized [2-4].Thin
layer chromatography (TLC) has been reported as a tool for reaction
optimization in microwave assisted synthesis [5]. This method has been used to
modify a conventional procedure for an efficient synthesis of metformin
hydrochloride by simply spotting of the reaction mixture on a TLC plate and
then subjecting it to microwave irradiation.
Experimental
In a typical experiment, a test
reaction was carried out on a 5×20 cm
TLC plate, a spot of solution of dicyanodiamide 1 (0.42 g) and dimethylamine
hydrochloride 2 (0.4 g) in 5 ml ethanol was placed on a TLC
plate and subjected to MWI at 540
W at
an interval of 40 seconds
intermittently for 5 min. Then, the TLC plate was run in an appropriate system.
A prominent spot of metformin hydrochloride
3 was seen and the rf value
of which was consistent with that of the
standard sample. In order to get an appreciable yield of pure product, the
reaction was carried out on a preparative TLC plate. An array of spots of
reactants for the synthesis of metformin hydrochloride was put on a preparative
TLC plate along with a reference TLC with two spots (one of the reactants and
other of expected product). Both the plates were subjected to MWI
intermittently at an interval of 40 s at 540 powers for 5 min. The reference TLC was viewed in an iodine chamber
and accordingly that portion of silica gel containing the product was scratched
from the preparative TLC plate and the product was extracted in EtOH. Evaporation
of the solvent afforded 0.82 g (92% yields) of the desired product.

The yield of metformin hydrochloride by this ecofriendly method was 92% and
the reaction time was 5 min. The
viability and uniqueness of this method can serve as a useful tool for rapid
reaction optimization in metformin hydrochloride synthesis.
The advantages of this ecologically safe protocol includes a simple
reaction set up that does not requires
specialized equipment, shorter reaction time, reduction of solvent, clean product,
optimum use of energy and usage of only few milligrams of reactants in a few
drops of solvent .
The financial support of the Iranian Research Organization for Science and
Technology is appreciated (Grant No. 1010278262).
© 2008 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.