Synthesis of New β-enaminones of Isoquinolines with 5,5-dimethyl-cyclohexanedione
Iliyan Ivanov and Stoyanka Nikolova
Department of Organic Chemistry, Faculty of Chemistry, Plovdiv University, 24 Tsar Assen str., Plovdiv, BG-4000, Bulgaria
Tel: +359 (32) 261 349, Fax: +359 (32) 628 392, e-mail: [email protected]
Received: 3 August 2007 / Accepted: 23 January 2008 / Published: 25 March 2008
Keywords: enaminones, dimedone, isoquinoline
Introduction
Enaminones are chemical compounds incorporating an amino group linked
through a C=C to carbonyl group. They
are versatile synthetic intermediates that combine the ambident
electrophilicity of enones with the ambident nucleophilicity of enamines. They
are typical push-pull systems in which the amine group pushes and the carbonyl
pulls electron density. The carbonyl group, conjugated with the enamine moiety,
gives this system enough stability to be easily prepared, isolated and stored
under atmospheric conditions at room temperature [1]. Enaminones are known to
possess a variety of medicinal properties including anticonvulsant,
antimalarial, antiinflamatory, and cardiovascular effects [2-6].
Even though numerous synthesis of enaminones have been reported, the
most common way for their preparation remains the condensation between an amine
and a 1,3-dicarbonyl compound [7]. In our previous work [8] we obtained β-enaminones of
primary amines by stirring the reaction mixture overnight at room temperature
or at reflux temperature in dichloroethane for 3h.
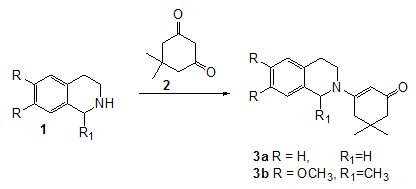
Now we wish to report the synthesis of new enaminones of secondary
amines, as 1,2,3,4-tetrahydroisoquinoline and
1-methyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline with
5,5-dimethyl-cyclohexanedione (dimedone). Enaminones of this type are more
difficult to obtain than enaminones of primary amines. The enaminones of
1,2,3,4-tetrahydroisoquinolines and dimedone were obtained by refluxing
equimolar amounts of the reagents for 3h in toluene using Dean-Stark trap to
remove the water formed during the reaction.
After completion of the reaction the solvent was removed by distillation.
Enaminones were purified by column chromatography on silica
gel 60 (Merck, 0.063-0.2mm) using n-hexane:Et2O=1:1 and Et2O
as eluent.
3-(3,4-Dihydro-1H-isoquinolin-2-yl)-5,5-dimethyl-cyclohex-2-enone (3a)
Melting Point: 103-105�C, 82% yield,
IR (KBr, cm-1): 1588;
1562
1H-NMR(600 MHz, CDCl3):
7.24-7.21(m,2H), 7.19-7.17(m,1H), 7.15-7.12(m,1H), 5.35(s,1H), 4.48(s,2H), 3.59(t,2H,J=5.9), 2.93(t,2H,J=5.9), 2.39(s,2H),
2.20(s,2H), 1.11(s,6H); 13C-NMR(150.9, CDCl3): 196.8,
163.0, 134.7, 132.8, 128.2, 127.2, 126.7, 126.4, 98.3, 49.4, 48.6, 44.4, 41.2,
33.0, 28.8.
Anal. Calcd for C17H21NO:
C 79.96, H 8.29, N 5.49. Found: C 80.08, H 8.32, N 5.53.
3-(6,7-Dimethoxy-1-methyl-3,4-dihydro-1H-isoquinolin-2-yl)-5,5-dimethyl-cyclohex-2-enone
(3b)
Melting Point: 197-200�C, 80% yield,
IR (KBr, cm-1): 1548;
1520
1H-NMR(600 MHz, CDCl3):
6.63(s,1H), 6.57(s,1H), 5.36(s,1H), 4.86(q,1H,J=6.5), 3.88(s,6H),
3.74-3.81(m,1H), 3.45-3.40(m,1H), 2.94-2.89(m,1H), 2.78(dt,1H,J=15.7,3.8),
2.43(d,1H,J=15.9), 2.29(d,1H,J=15.9), 2.22(d,1H,J=16.2), 2.16(d,1H,J=16.2),
1.50(d,3H,J=6.6), 1.13(s,3H), 1.08(s,3H); 13C-NMR(150.9, CDCl3):
196.6, 162.0, 147.9, 147.8, 129.9, 129.8, 111.2, 109.4, 97.8, 56.1, 55.9, 52.8,
49.4, 41.0, 40.7, 30.1, 27.6.
Anal. Calcd for C20H27NO3:
C 72.92, H 8.26, N 4.25. Found: C 73.13, H 8.39, N 4.38.
Acknowledgment
The authors acknowledge financial
support from the Fund for scientific research of the Plovdiv University.
References
- Kascheres,
C. J. Braz. Chem. Soc. 2003, 14, 945.
- Eddington,
N., Cox, D., Roberts, R., Stables, J., Powell, C., Scott, K. Curr. Med. Chem. 2000, 7, 417.
- Scott, K., Rankin, G. Stables, J.,
Alexander, M., Edafiogho, I., Farrar, V., Kolen, K., Moore, J., Sims, L.
and Tonnut, A. J. Med. Chem. 1995, 38, 4033.
- Foster, J., Nicholson, J., Butcher, R.,
Stables, J., Edafiogho, I., Goodwin, A., Henson, M., Smith, C., Scott, K. Bioorg. Med. Chem. 1999, 7, 2415.
- Edafiogho, I., Ananthalakshmi, K.,
Kombian, S. Bioorg. Med. Chem. 2006, 14, 5266.
- Ananthalakshmi, K., Edafiogho, I.,
Kombian, S. Neuroscience 2006, 141, 345.
- Venkov,
A., Angelov, P. Synt.Comm. 2003, 33, 3025.
- Ivanov, I., Nikolova, St., Angelov,
P.,
Statkova-Abeghe, St., Kochovska, E. Arkivoc 2007, 15, 11.
�
2008 by MDPI (http://www.mdpi.org/).
Reproduction is permitted for noncommercial purposes.