|
Molbank 2008 M566 |
N-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl (N-Dde) Lipoamino Acids
Benjamin P. Ross 1, Robert A. Falconer2 and Istvan Toth 1,3,*
1 School of Pharmacy, The University of Queensland, Brisbane, Queensland 4072, Australia.
2 Institute of Cancer Therapeutics, School of Life Sciences, University of Bradford, West Yorkshire, BD7 1DP, United Kingdom.
3 School of Molecular and Microbial Sciences, The University of Queensland, Brisbane, Queensland 4072, Australia.
*Author to whom correspondence should be addressed; Tel.: +61 7 3346 9892; Fax: +61 7 3365 4273; E-mail: [email protected]
Received: 28 March 2007 / Accepted: 7 February 2008 / Published: 15 August 2008
Keywords: lipoamino acid, LAA, 2-aminoalkanoic acid, 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl, Dde.
The conjugation of a drug with one or more lipoamino acids (LAAs; 2-aminoalkanoic acids; e.g. 3) can impart upon the drug steric protection from enzymatic degradation, increased lipophilicity, increased passive membrane diffusion and therefore may improve drug bioavailability [1]. A variety of small molecules have been conjugated with LAAs [2], and many promising conjugates are peptides [3, 4]. N-tert-Butoxycarbonyl (N-Boc) LAAs are commonly used to introduce the LAA moiety into a peptide [3], however, N-Boc LAAs are incompatible with the popular 9-fluorenylmethoxycarbonyl (Fmoc) strategy of solid-phase peptide synthesis [5-7]. N-Fmoc LAAs have been prepared, but these compounds were poorly soluble in most organic solvents and therefore difficult to handle [8]. The stability of the N-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl (N-Dde) group to both acidic and some basic conditions [9], and its facile removal by hydrazine or hydroxylamine [9-11], have led to its successful incorporation into many solid-phase methodologies [9, 10, 12-14]. Kellam et al. [15] used a N-Dde LAA (4c) in the synthesis of a glycolipopeptide by Fmoc-chemistry, however, 4c was only partially characterized. Malkinson et al. [16, 17] used N-Dde LAAs in the synthesis of somatostatin analogues, but the preparation of the N-Dde LAAs was not described. Herein we report the spectra of 4c and the novel homologues 4a and 4b, which were prepared by a method similar to that of Kellam et al. [15].
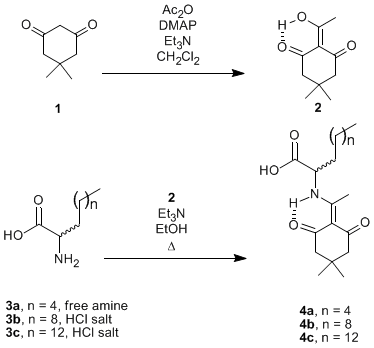
2-Acetyldimedone (2) was synthesized by acylation of dimedone (1) with acetic anhydride. In a modification to the procedures that require one equivalent of 4-dimethylaminopyridine (DMAP) [18-20], it was found that purification was easier and by-product formation less significant when triethylamine was used as the base in the presence of a catalytic quantity of DMAP. The N-Dde LAAs (4a-c) were prepared by reaction of the LAAs (3a-c) with 2-acetyldimedone (2) in the presence of triethylamine in refluxing ethanol.
2-Acetyldimedone (2)
Dimedone (1, 15.0 g, 107 mmol) was dissolved in CH2Cl2 (100 mL). DMAP (2.61 g, 21.4 mmol) and triethylamine (29.8 mL, 214 mmol) were added and the mixture was stirred for 10 min. Acetic anhydride (12.2 mL, 129 mmol) was added and the mixture was stirred under an atmosphere of argon for 48 h. The solvent was removed by co-evaporation with toluene and the residue was dissolved in ethyl acetate (300 mL) and washed with 5% HCl (3 ´ 300 mL), dried (MgSO4), filtered, and evaporated in vacuo to produce an oil. This oil was filtered through a column of silica (silica gel 60, 230-400 mesh, ethyl acetate:n-hexane, 2:3) and the solvent was evaporated to afford the title compound (2) as pale yellow crystals (11.3 g, 58%).
Melting point 35-36 °C (literature melting point 36-38 °C [21]).
ESI-MS, m/z: 183 [M + H]+.
1H NMR (500 MHz, CDCl3) d 18.02 (1H, s, H-bonded OH), 2.54 (3H, s, CH3), 2.48 (2H, s, CH2), 2.30 (2H, s, CH2), 1.02 [6H, s, C(CH3)2].
13C NMR (125 MHz, CDCl3) d 202.2, 197.7, 194.9, 112.2, 52.3, 46.8, 30.5, 28.3, 28.0.
2-[(4,4-Dimethyl-2,6-dioxocyclohex-1-ylidene)ethylamino]-D,L-octanoic Acid (4a)
2-Amino-D,L-octanoic acid [22, 23] (3a, 5.67 g, 35.6 mmol) and 2-acetyldimedone (2, 7.14 g, 39.2 mmol) were suspended in ethanol (200 mL). Triethylamine (5.40 g, 7.44 mL, 53.4 mmol) was added and the mixture was refluxed under an atmosphere of argon for 18 h. The solvent was removed in vacuo and the residue was taken up in ethyl acetate (250 mL) and washed with 5% HCl solution (3 ´ 200 mL). The organic layer was dried (MgSO4), filtered and evaporated in vacuo to give a pale yellow solid which was triturated with diethyl ether to afford the title compound (4a) as a white solid (6.16 g, 54%).
Melting point 156-158 °C.
ESI-MS, m/z: 324 [M + H]+.
1H NMR (500 MHz, CDCl3) d 13.69 (1H, d, J 7.3 Hz, H-bonded NH), 11.18 (1H, br s, COOH), 4.37 (1H, m, a-CH), 2.52 (3H, s, C(NH)CH3), 2.39 (4H, s, 2CH2CO), 2.02-1.87 (2H, m, b-CH2), 1.45-1.21 (8H, m, 4CH2), 1.01 [6H, s, C(CH3)2], 0.84 (3H, t, J 6.8 Hz, CH3).
13C NMR (125 MHz, CDCl3) d 198.9, 174.0, 172.1, 108.0, 56.5, 52.3, 32.5, 31.3, 30.1, 28.7, 28.1, 25.2, 22.4, 18.7, 13.9.
HRMS calcd for [M + H]+ 324.2174, found 324.2165; calcd for [M + Na]+ 346.1994, found 346.1999.
2-[(4,4-Dimethyl-2,6-dioxocyclohex-1-ylidene)ethylamino]-D,L-dodecanoic Acid (4b)
2-Amino-D,L-dodecanoic acid hydrochloride [24] (3b, 12.3 g, 48.7 mmol) and 2-acetyldimedone (2, 9.76 g, 53.5 mmol) were suspended in ethanol (200 mL). Triethylamine (12.3 g, 17.0 mL, 122 mmol) was added and the mixture was refluxed under an atmosphere of argon for ~32 h. The solvent was removed in vacuo and the residue was taken up in ethyl acetate (250 mL) and washed with 5% HCl solution (3 ´ 200 mL). The organic layer was dried (MgSO4), filtered and evaporated in vacuo to give a pale yellow solid which was triturated with diethyl ether to afford the title compound (4b) as a white solid (10.0 g, 54%).
Melting point 110-112 °C.
ESI-MS, m/z: 380 [M + H]+.
1H NMR (500 MHz, CDCl3) d 13.70 (1H, d, J 7.2 Hz, H-bonded NH), 11.74 (1H, br s, COOH), 4.37 (1H, m, a-CH), 2.52 [3H, s, C(NH)CH3], 2.39 (4H, s, 2CH2CO), 2.02-1.87 (2H, m, b-CH2), 1.43-1.23 (16H, m, 8CH2), 1.01 [6H, s, C(CH3)2], 0.85 (3H, t, J 6.9 Hz, CH3).
13C NMR (125 MHz, CDCl3) d 198.9, 174.0, 172.1, 108.0, 56.5, 52.3, 32.6, 31.8, 30.1, 29.4, 29.2, 29.1, 28.2, 25.3, 22.6, 18.7, 14.0.
HRMS calcd for [M + H]+ 380.2801, found 380.2802.
2-[(4,4-Dimethyl-2,6-dioxocyclohex-1-ylidene)ethylamino]-D,L-hexadecanoic Acid (4c)
Compound 4c, a white solid, was prepared from 2-amino-D,L-hexadecanoic acid hydrochloride [22] by following the procedure described for 4b: scale 38.9 mmol; yield 6.61 g (39%).
Melting point 101-105 °C (literature melting point 115-117 °C [15]).
ESI-MS, m/z: 436 [M + H]+.
1H NMR (500 MHz, CDCl3) d 13.70 (1H, d, J 7.3 Hz, H-bonded NH), 10.7 (1H, br s, COOH), 4.37 (1H, m, a-CH), 2.52 [3H, s, C(NH)CH3], 2.39 (4H, s, 2CH2CO), 2.01-1.88 (2H, m, b-CH2), 1.43-1.23 (24H, m, 12CH2), 1.02 [6H, s, C(CH3)2], 0.85 (3H, t, J 6.9 Hz, CH3).
13C NMR (125 MHz, CDCl3) d 199.0, 174.1, 172.3, 108.2, 56.7, 52.5, 52.4, 32.7, 32.0, 30.2, 29.8, 29.7, 29.7, 29.6, 29.4, 29.4, 29.3, 28.3, 28.2, 25.5, 22.7, 18.8, 14.2.
HRMS calcd for [M + H]+ 436.3427, found 436.3426.
Acknowledgments
We thank Mr Graham MacFarlane (School of Molecular and Microbial Sciences, The University of Queensland) for accurate mass measurements.
References
- Wong, A.; Toth, I. Curr. Med. Chem. 2001, 8, 1123-1136. Blanchfield, J.; Toth, I. Curr. Med. Chem. 2004, 11, 2375-2382.
- Recent examples include: Pignatello, R.; Jansen, G.; Kathmann, I.; Puglisi, G.; Toth, I. J. Pharm. Sci. 1998, 87, 367-371. Pignatello, R.; Vicari, L.; Sorrenti, V.; Di Giacomo, C.; Spampinato, G.; Puglisi, G.; Toth, I. Drug Dev. Res. 2001, 52, 454-461. Wimmer, N.; Robinson, J. A.; Gopisetty-Venkatta, N.; Roberts-Thomson, S. J.; Monteith, G. R.; Toth, I. Med. Chem. 2006, 2, 79-87. Pignatello, R.; Guccione, S.; Castelli, F.; Sarpietro, M. G.; Giurato, L.; Lombardo, M.; Puglisi, G.; Toth, I. Int. J. Pharm. 2006, 310, 53-63. Kim, I.-H.; Nishi, K.; Tsai, H.-J.; Bradford, T.; Koda, Y.; Watanabe, T.; Morisseau, C.; Blanchfield, J.; Toth, I.; Hammock, B. D. Bioorg. Med. Chem. 2007, 15, 312-323.
- Recent examples include: Benson, H. A. E.; Caccetta, R.; Chen, Y.; Kearns, P.; Toth, I. Lett. Pept. Sci. 2004, 10, 615-620. Blanchfield, J. T.; Lew, R. A.; Smith, A. I.; Toth, I.; Bioorg. Med. Chem. Lett. 2005, 15, 1609-1612. Caccetta, R.; Blanchfield, J. T.; Harrison, J.; Toth, I.; Benson, H. A. E. Int. J. Pept. Protein Res. Ther. 2006, 12, 327-333. Amon, M. A.; Ali, M.; Bender, V.; Chan, Y.-N.; Toth, I.; Manolios, N. Biochem. Biophys. Acta 2006, 1763, 879-888. See also [15, 16, 24].
- Recent examples include: Wong, A. K.; Ross, B. P.; Chan, Y.-N.; Artursson, P.; Lazorova, L.; Jones, A.; Toth, I. Eur. J. Pharm. Sci. 2002, 16, 113-118. Dulhunty, A. F.; Cengia, L.; Young, J.; Pace, S. M.; Harvey, P. J.; Lamb, G. D.; Chan, Y.-N.; Wimmer, N.; Toth, I.; Casarotto, M. G. Br. J. Pharm. 2005, 144, 743-754. Wu, S.; Campbell, C.; Koda, Y.; Blanchfield, J. T.; Toth, I. Med. Chem. 2006, 2, 203-211.
- Atherton, E.; Fox, H.; Harkiss, D.; Logan, C. J.; Sheppard, R. C.; Williams, B. J. J. Chem. Soc. Chem. Commun. 1978, 537.
- Chang, C. –D.; Meienhofer, J. Int. J. Pept. Protein Res. 1978, 11, 246.
- Wellings, D. A.; Atherton, E. In Methods in Enzymology, Academic Press: 1997; Vol. 289, pp 44-67.
- Toth, I. Unpublished results.
- Bycroft, B. W.; Weng, C. C., Chhabra, S. R.; Hone, N. D. J. Chem. Soc. Chem. Commun. 1993, 778-779.
- Diaz-Mochon, J. J.; Bialy, L.; Bradley, M. Org. Lett. 2004, 6, 1127-1129.
- Typically, 2% v/v hydrazine in N,N-dimethylformamide [9], or a mixture of NH2OH.HCl/imidazole in N-methyl-2-pyrrolidone/CH2Cl2 which is fully orthogonal to Fmoc [10].
- Kellam, B.; Bycroft, B. W.; Chhabra, S. R. Tetrahedron Lett. 1997, 38, 4849-4852.
- Sharma, S. K.; Wu, A. D.; Chandramouli, N.; Fotsch, C.; Kardash, G.; Bair, K. W. J. Pept. Res. 1999, 53, 501-506.
- Connolly, P. J.; Beers, K. N.; Wetter, S. K.; Murray, W. V. Tetrahedron Lett. 2000, 41, 5187-5191.
- Kellam, B.; Drouillat, B.; Dekany, G.; Starr, M. S.; Toth, I. Int. J. Pharm. 1998, 161, 55-64.
- Toth, I.; Malkinson, J. P.; Flinn, N. S.; Drouillat, B.; Horvath, A.; Erchegyi, J.; Idei, M.; Venetianer, A.; Artursson, P.; Lazorova, L.; Szende, B.; Keri, G. J. Med. Chem. 1999, 42, 4010-4013.
- Malkinson, J. P.; Lazorova, L.; Artursson, P.; Gyoergy, K.; Toth, I.; Peptides 2000, Proceedings of the 26th European Peptide Symposium, Montpellier, France, September 10-15, 2000; Editions EDK: Paris, France, 2001; pp 319-320.
- Kellam, B.; Weng, C. C.; Chhabra, S. R.; Bycroft, B. W. Tetrahedron Lett. 1997, 38, 5391-5394.
- Chhabra, S. R.; Hothi, B.; Evans, D. J.; White, P. D.; Bycroft, B. W.; Chan, W. C. Tetrahedron Lett. 1998, 39, 1603-1606.
- Portal, C.; Launay, D.; Merritt, A.; Bradley, M. J. Comb. Chem. 2005, 7, 554-560.
- Forsen, S.; Nilsson, M. Acta Chemica Scandinavica 1959, 13, 1383-1394.
- Ross, B. P.; Braddy, A. C.; McGeary, R. P.; Blanchfield, J. T.; Prokai, L.; Toth, I. Mol. Pharmaceutics 2004, 1, 233-245.
- Gibbons, W. A.; Hughes, R. A.; Charalambous, M.; Christodoulou, M.; Szeto, A.; Aulabaugh, A. E.; Mascagni, P.; Toth, I. Liebigs Ann. Chem. 1990, 1175-1183.
- Blanchfield, J. T.; Dutton, J. L.; Hogg, R. C.; Gallagher, O. P.; Craik, D. J.; Jones, A.; Adams, D. J.; Lewis, R. J.; Alewood, P. F.; Toth, I. J. Med. Chem. 2003, 46, 1266-1272.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland.
This
article is an open-access article distributed under the terms and
conditions of the Creative Commons Attribution license
(http://creativecommons.org/licenses/by/3.0/).