Molbank 2008, M569
![]()
molbank
ISSN 1422-8599
www.mdpi.org/molbank
Short Note
Synthesis
of 4,4'-(Cyclohexane-1,1-diyl)bis(1-methyl-1H-pyrazol-5-ol)
Gernot A. Eller * and Wolfgang Holzer
Department of Drug and Natural
Product Synthesis,
* Author to whom correspondence
should be addressed.
Received: 1 July 2008 / Accepted: 19 August 2008 / Published: 22 August 2008
Abstract: Methyl
(2E)-3-methoxyacrylate and excess
methylhydrazine yield crude 1-methyl-2-pyrazolin-5-one which is reacted with
cyclohexanone to obtain the title compound in 86% yield. Detailed spectroscopic
data (1H NMR, 13C NMR, 15N NMR, and MS) are
presented.
Keywords: Pyrazolones, cyclohexanone adduct, NMR
spectroscopy.
1. Introduction
During our ongoing research on pyrano[2,3-c]pyrazol-4(1H)-ones [1每5]
we were interested in the not yet commercially available methylpyrazolone 1. Although dozens of patents and
journal articles deal with its synthesis, most of them include multi-step
reactions or seem to be less suited for laboratory scale preparations. Thus, we
chose to follow a patent protocol, which starts from alkoxyacrylic alkyl esters
and alkylhydrazines [6]. Recently, we have successfully applied that procedure to
yield the unsubstituted pyrazolone [7]. Unfortunately, no work up for the crude
methylpyrazolone 1 is published in
the original patent (only GC/MS analysis is carried out). When we tried to find
a proper solvent to precipitate the desired methylpyrazolone from the oily
reaction syrup, we found that ketones 每 such as acetone or cyclohexanone 每 readily
react and give colourless precipitates as the analysis of such a precipitate
revealed. A representative procedure with cyclohexanone is presented in the
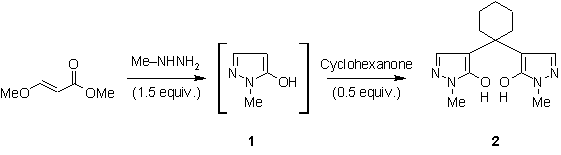
experimental part [Scheme 1].
Scheme 1. Synthesis
of the title compound 2
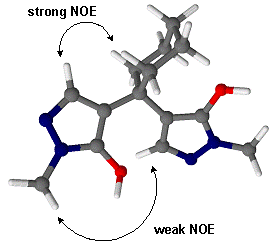
It should be noted that according to NMR data in DMSO-d6 solution, we presume a
rather unsymmetrical conformation as outlined in Scheme 2. Consistent with this
suggestion, we achieved no intramolecular cyclization to the corresponding
pyrano[2,3-c:6,5-c']dipyrazole upon treatment with conc. sulfuric acid or
polyphosphoric acid, that would be expected if the hydroxy groups were
spatially close.
Scheme 2. Proposed conformation of compound 2 in DMSO solution [8]
2. Experimental
Melting points were determined on a Reichert每Kofler hot-stage microscope
and are uncorrected. Mass spectra were obtained on a Shimadzu QP 1000
instrument (EI, 70 eV). Elemental analysis was performed at the Microanalytical
Laboratory,
2.1. 4,4'-(Cyclohexane-1,1-diyl)bis(1-methyl-1H-pyrazol-5-ol)
(2)
CAUTION: Methylhydrazine is a potentially highly toxic
compound and must be used with great care under a well-ventilated hood.
To a well stirred solution of methyl 3-methoxyacrylate (50 mmol, 5.80 g)
in dry MeOH (5 mL) methylhydrazine (75 mmol, 3.46 g) was added dropwise and the
mixture was stirred at room temperature for 3 h. Then the excess solvent and
reagents were distilled off using a rotary evaporator to obtain the crude
methylpyrazolone 1 as a yellowish
honey-like mass [9]. Cyclohexanone (25 mmol, 2.45 g) was added to the residue
and the mixture was refluxed for 5 min. The formed precipitate was filtered off
and washed subsequently with petroleum ether and acetone to yield the pure
title compound 2 (5.91 g, 86%).
Mp: 186每191 ∼C, colourless powder.
1H NMR (300 MHz, DMSO-d6): 汛 (ppm) 13.77 (br s, 2H, OH), 7.16 (s, 2H, pyrazole
H-3), 3.38 (s, 6H, NMe), 2.07 (m, 4H, cyclohexane H-2,6), 1.38 (m, 6H,
cyclohexane H-3,4,5).
13C NMR (75 MHz, DMSO-d6): 汛 (ppm) 156.0 (pyrazole C-5), 132.6 (pyrazole C-3, 1J = 183.8 Hz), 110.9 (pyrazole C-4),
33.5 (cyclohexane C-1), 33.2 (cyclohexane C-2,6), 31.6 (NMe, 1J = 140.0 Hz), 26.0 (cyclohexane C-4),
21.9 (cyclohexane C-3,5).
15N NMR (50 MHz, DMSO-d6): 汛 (ppm) 每210.0 (N-1); N-2 was not found.
MS (m/z,
%): 276 (M+, 3), 179 (42), 178 (100), 163 (50), 149 (34), 135 (33),
124 (28), 111 (48), 99 (26), 98 (53), 79 (36), 55 (45), 43 (46).
Elemental Analysis: Calculated for C14H20N4O2
(276.33): C, 60.85%; H, 7.30%; N, 20.28%. Found: C, 60.66%; H, 7.24%; N, 20.34%.
References and Notes
1. Eller,
G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An efficient approach to
heterocyclic analogues of xanthone: a short synthesis of all possible
pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 24, 4219.
2. Eller,
G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri-
and tetracyclic heteroaromatic systems: synthesis of novel benzo-, benzothieno-
and thieno-fused pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87.
3. Eller, G.A.; Holzer, W. A
convenient approach to heterocyclic building blocks: synthesis of novel ring
systems containing a [5,6]pyrano[2,3-c]pyrazol-4(1H)-one moiety. Molecules 2007, 12, 60.
4. Eller,
G.A.; Datterl, B.; Holzer, W. Pyrazolo[4',3':5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: synthesis and characterization of a novel tetracyclic ring
system. J. Heterocycl. Chem. 2007, 44, 1139.
5. Eller,
G.A.; Wimmer, V.; Holzer, W. Synthesis of novel polycyclic ring
systems containing two pyrano[2,3-c]pyrazol-4(1H)-one moieties. Khim.
Geterotsikl. Soedin. 2007, 1251; Chem. Heterocycl. Comp. 2007, 43, 1060.
6. Maywald,
V.; Steinmetz, A.; Rack, M.; Gotz, N.; Gotz, R.; Henkelmann, J.; Becker, H.;
Aiscar Bayeto, J.J. Preparation of 1-substituted 5- or 3-hydroxypyrazoles from
alkoxyacrylates and hydrazines. PCT Int. Appl. WO 0031042, 2000; [Chem.
Abstr., 2000, 133, 4655].
7. Eller, G.A.;
Holzer, W. A one-step synthesis of pyrazolone. Molbank 2006,
M464; Chem. Abstr. 2006,
145, 505356
8. ACD/3D
Viewer, version 10.00; Advanced Chemistry Development, Inc.:
9. Upon
trituration and subsequent recrystallization of the oily residue with/from i.e.
diethyl ether, pure methylpyrazolone 1
can be obtained as colourless crystals. Mp 108.5每111 ∼C. 1H NMR (500
MHz, DMSO-d6): 汛 (ppm) 10.90
(br s, 1H, OH), 7.09 (d, 3J(H-3,H-4)
= 1.9 Hz, 1H, H-3), 5.30 (d, 3J(H-4,H-3)
= 1.9 Hz, 1H, H-4), 3.47 (s, 3H, NMe). 13C NMR (125 MHz, DMSO-d6): 汛 (ppm) 152.6 (C-5, 2J(C-5,H-4) = 5.7 Hz, 3J(C-5,H-3) = 10.5 Hz, 3J(C-5,NMe) = 1.9 Hz), 137.1 (C-3, 1J = 182.7 Hz, 2J(C-4,H-3) = 5.1 Hz), 86.1 (C-4, 1J = 176.1 Hz), 32.9 (NMe, 1J = 139.3 Hz). 15N NMR (50
MHz, DMSO-d6): 汛 (ppm) −201.9
(N-1); N-2 was not found.
Sample Availability: Compounds 1 and 2 are available
from MDPI.
© 2008 by the
authors; licensee Molecular Diversity Preservation International,