Molbank 2008, M579
![]()
molbank
ISSN 1422-8599
www.mdpi.org/molbank
Short Note
Determination of the Absolute Configurations of (+)-N-((3S)-3-{[(4-methylphenyl)sulfonyl]amino}-1-oxaspiro[4.5]deca-6,9-dien-2,8-dion-7-yl) Acetamide and Benzamide
1 University of Northern British Columbia, Department of Chemistry, 3333 University Way, Prince George, British Columbia, V2N 4Z9, Canada
2 College of the Rockies, University Studies (Chemistry), Box 8500, Cranbrook, British Columbia, V1C 5L7, Canada
* Author to whom correspondence should be addressed; E-mail: [email protected]; Tel. +1-250-960-6694; Fax: +1-250-960-5845
Received: 18 September 2008 / Accepted: 3 November 2008 / Published: 7 November 2008
Abstract: We recently reported the asymmetric synthesis of the two title compounds without the configurational assignments of the newly formed chiral spirocarbons. We now wish to report that both compounds have a (R)-configuration at the spirocarbon based on 1D and 2D nuclear Overhauser enhancement (nOe) experiments.
Keywords: Spiroannulation, stereochemistry, nOe, NMR, absolute configuration.
1. Discussion
For the past few years we have studied the diastereoselective spiroannulation of simple phenols [1-11], and we recently reported the asymmetric synthesis of two new spirolactones (+)-1 and (+)-2 (Figure 1) from optically active (S)-3-nitrotyrosine [1]. However, at the time of publication we had yet to determine the absolute configuration of the newly formed spirocentre in (+)-1 and (+)-2. We now wish to report the absolute configuration of these two compounds as determined using one- and two-dimensional nuclear Overhauser enhancement [nOe] NMR methods. In the absence of crystals suitable for X-ray analysis, we felt that nOe techniques would be the best way to determine these configurations. We believe that such assignments can be made using NMR techniques since the structure of the two spirocompounds is rigid at the spirocarbon and the stereochemistry of carbon 3 of the lactone ring is known to have a (S)-configuration.
The two possible diastereomers of (+)-1 and (+)-2 are shown in Figure 1 (structures A and B). Since carbon 3 in the lactone ring has a (S)-configuration as shown in Figure 1 [12], irradiation of H3 should affect only one of H10 (structure A) or H6 (structure B) assuming that these protons are in close enough proximity to H3 to be affected. It is normally assumed that 1H nOe can be observed between protons located within 500 pm (5Ǻ) of each other [13,14]. This is about twice the distance separating 1,3-diaxial protons on the chair form of cyclohexane (~2.6Ǻ) [14]. When comparing models of cyclohexane with either structures A or B, we estimated that the distance between H3 and either H10 or H6 falls within the range normally expected to observe nOe [15].
Figure 1. Possible Configurations for (+)-1 and (+)-2
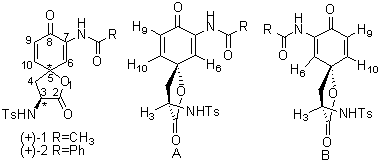
Figure 2. Portion of the Original 1H-NMR spectrum of (+)-2 in CDCl3
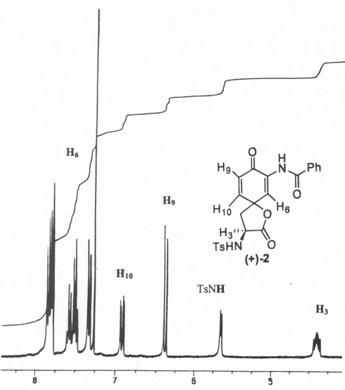
Figure 2 shows a portion of the original 1H-NMR spectrum of (+)-2 while nOe results are summarized in Table 1. Irradiation was carried out on all three protons (H3, H6 and H10) with a 2s presaturation time for each experiment. 1D nOe studies of (+)-1 showed that irradiation of H3 produced enhancement of the signal of H10 (7%) while no nOe effect was observed for H6. The reverse experiment, i.e. irradiation of H10, showed nOe effect on H3 (6%) as well as enhancement of the signal of H9 (5%). No effect was observed between H3 and H6 when either H3 or H6 were irradiated. This data suggests that the correct configuration for the spirocarbon of (+)-1 is as shown in structure A found in Figure 1, in other words the spirocarbon has a (R)-configuration. A similar analysis can be performed for (+)-2.
Table 1. Data from nOe Experiments and Chemical Shifts of Key Protonsa.
|
Compound |
Irradiation |
Integration |
||||
|
|
|
H10 |
H9 |
H6 |
H3 |
|
|
|
H10 (7.06ppm) |
1.00 |
0.05 |
n/a |
0.06 |
|
|
(+)-1 |
H6 (7.52ppm) |
n/a |
n/a |
1.00 |
n/a |
|
|
|
H3 (4.54ppm) |
0.07 |
n/a |
n/a |
1.00 |
|
|
|
H10 (6.92ppm) |
1.00 |
0.04 |
n/a |
0.05 |
|
|
(+)-2 |
H6 (7.54ppm) |
n/a |
n/a |
1.00 |
n/a |
|
|
|
H3 (4.40ppm) |
0.04 |
n/a |
n/a |
1.00 |
|
aChemical shifts listed are reported from ref. 1. n/a: no signal visible in nOe difference spectrum
Two dimensional nOe experiments (NOESY and ROESY) were also performed for (+)-1 and (+)-2, and confirmed our results obtained by 1D difference nOe studies. In these experiments, correlation between H10 and H3 for both compounds was observed while correlation between H3 and H6 was absent, confirming that structure A is the correct structure for both (+)-1 and (+)-2.
Based on these studies, we can conclude that the chiral spirocarbons in both (+)-1 and (+)-2 have a (R)-configuration as depicted by structure A in Figure 1. We are still attempting to obtain crystals of the target compounds suitable for X-ray analysis in order to unambiguously assign the configurations of these two compounds.
2. Experimental
1- and 2-dimensional nOe experiments were carried out on a Bruker 300AMX spectrometer at a frequency of 300.13 MHz. Samples were dissolved in CDCl3 and the spectra were referenced to the residual solvent signal (CHCl3) at 7.26 ppm. Samples were only slightly soluble in CDCl3 but stable. Samples were not degassed prior to data accumulation. 1D experiments were performed with a 2s presaturation time, 1024 scans were recorded. Mixing times for the ROESY and NOESY experiments were 200ms and 300ms respectively.
Acknowledgement
We acknowledge the financial contribution of the University of Northern British Columbia in support of this work.
References and Notes:
1. Plourde, G.L.; Spaetzel, R.R.; Kwasnitza, J.S.; Scully, T.W. Diastereoselective Spiroannulation of Phenolic Substrates: Advances Towards the Asymmetric Formation of the Manumycin m-C7N Core Skeleton. Molecules 2007, 12, 2215-2222.
2. Plourde, G.L. Studies Towards the Diastereoselective Spiroannulation of Phenolic Derivatives. Tetrahedron Letters 2002, 43, 3597-3599.
3. Plourde, G.L. (±)-1-(4-Hydroxy-3-methoxyphenyl)-3-butanol. Molbank, 2003, M315.
4. Plourde, G.L. (±)-7-Methoxy-2-methyl-1-oxaspiro[4,5]deca-6,9-diene-8-one. Molbank, 2003, M316.
5. Plourde, G.L. 1-(4-Hydroxy-3-methoxyphenyl)-4-methyl-3-pentanone. Molbank, 2003, M317.
6. Plourde, G.L. (±)-1-(4-Hydroxy-3-methoxyphenyl)-4-methyl-3-pentanol. Molbank, 2003, M318.
7. Plourde, G.L. (±)-7-Methoxy-2-ipropyl-1-oxaspiro[4,5]deca-6,9-diene-8-one. Molbank, 2003, M319.
8. Plourde, G.L. 1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanone. Mobank, 2003, M320.
9. Plourde, G.L. (±)-1-(4-Hydroxy-3-methoxyphenyl)-4,4-dimethyl-3-pentanol. Molbank, 2003, M321.
10. Plourde, G.L. (±)-2-tButyl-7-methoxy-1-oxaspiro[4,5]deca-6,9-diene-8-one. Mobank, 2003, M322.
11. Plourde, G.L.; English, N.J. Diastereoselective Spiroannulation of Phenolic Substrates: Synthesis of (±)-2-tert-Butyl-6-methoxy-1-oxaspiro[4,5]deca-6,9-diene-8-one. Molecules 2005, 10, 1335-1339.
12. The configuration of the chiral centre at carbon 3 in (+)-1 and (+)-2 should be the same as the original chiral centre in (S)-3-nitrotyrosine under the reactions conditions used in the synthesis reported [(a) 1) TsCl, THF, 1M NaOH 2) 1M KOH, EtOH, 80-85 oC; (b) H2, 10% Pd/C, THF; (c) CH3COCl or PhCOCl, THF, rt; (d) PIFA, acetone, 0oC] (reference 1). Therefore, it is assumed that this centre remained in the (S)-configuration.
13. Eliel E.L.; Wilen S.H. Stereochemistry of Organic Compounds; John Wiley & Sons, Inc.: New York, 1994; pp. 30-31.
14. Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th Edition; John Wiley & Sons, Inc.: New York, 1998; pp. 189-191.
15. The distance between H3 and H10/H6 is estimated to be between 2.6Ǻ and 5Ǻ. The actual distance between these protons in a solution will depend on molecular movement and steric factors found in the molecule. This distance has not been calculated.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).