1Molecular Diversity Preservation International (MDPI), Matthaeusstrasse 11, CH-4057 Basel, Switzerland. Tel. +41 79 322 3379, Fax +41 61 302 8918, e-mail: [email protected], http://www.mdpi.org/lin
2Institute of Organic Chemistry, BCH, University of Lausanne,
CH-1015 Lausanne, Switzerland.
Tel. +41 21 692 40 69, Fax +41 21 692 39 55, e-mail: [email protected]
3Advanced Chemistry Development Inc., ACD Inc., Moscow, ul. Akademika Bakuleva,6, str.1, ACD, Moscow 117513, Russia. e-mail: [email protected], http://www.acdlabs.com
4Beilstein Information, Frankfurt/M, Germany. Tel. +49-69-5050 4276, Fax +49-69-5050 4284, e-mail: [email protected], http://www.beilstein.com
5Institute of Medicinal Chemistry, BEP, University of Lausanne, CH-1015 Lausanne, Switzerland, Tel +41 21 692 4521, Fax +41 21 692 4525, e-mail [email protected]
*the corresponding author. E-mail: [email protected]
http://www.mdpi.org/molecules/wedge.htm
12 June 2000
Summary
We propose that various bonds (see figure below) used for specification of absolute configuration, e.g. the two types (I and II) of solid wedge and broken wedge representation most frequently seen in literature, can be replaced by only one kind of wedge, namely the solid wedge (III and IV). Only one wedge should be used when representing a quadrivalent center (IV). The three normal bonds are distributed on a cone opposite to the wedge. The flexibility, simplicity, unambiguity and usefulness for R-S specification of the one-wedge system are discussed, as well as its esthetic appeal.

Keywords: One-wedge convention, stereochemical representations,
configurational drawings, stereochemistry, stereogenic center, center of
chirality.
Introduction
Establishing a convention for stereochemical representation has become an urgent issue in recent years with the development of chemical information technology, particularly chemical structure database [1].
In 1858, Archibald Scott Couper [2] drew the first structural formula. In the following year, August Kekulé found that the atom carbon is quadrivalent, and he also set up the basic principle of structural determination. In 1874 [3] van't Hoff and Lebel independently deduced that the carbon atom is tetrahedral. Their inference was later abundantly confirmed by chemical and physical evidence. Stereochemistry was born. From there, progress has been steady, but with it arose problems in nomenclature and representation.
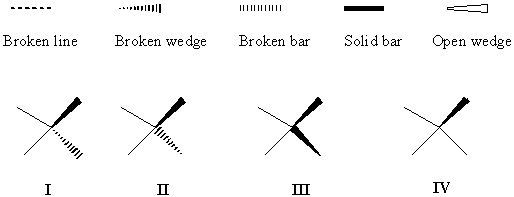
Besides Fisher and Newman projections used for special cases, the solid wedge, broken (or dashed, as some call it) wedge, broken line, solid bar, broken bar and open wedge are all frequently used in structural drawing (Figure 1) [4-8]. A large number of their combinations can be seen in the literature and in drawing softwares (ChemDraw, ISIS/Draw, Beilstein SE, ACD/ChemSketch, etc.), causing confusion and ambiguity, particularly in chemical structure database handling, as repeatedly noted [9-11]. For drawing conventions supported by Chemical Abstracts Service, see [4]. Wedge projection is a stereochemical projection, roughly in the mean plane of the molecule, in which bonds are represented by open wedges, tapering off from the nearer atom to the farther atom [5] and is mainly used to illustrate the conformation of larger cycloalkanes e.g. cyclotetradecane (www.chem.qmw.ac.uk/iupac/stereo/TZ.html). Zig-zag projection is a stereochemical projection for an acyclic molecule (or portion of a molecule) where the main chain is represented by a zig-zag line in the plane and the substituents are shown above or below the plane. A recent survey by Juaristi and Welch [6] found a significant number of stereochemists in favor of the use of bars - either a broken bar or a solid bar. The open wedges have been used for an entirely different purpose, namely, as the up bonds replacing the solid wedge, to distinguish between relative and absolute configuration (for example, see [7]).
For example, there are many structures in the frequently used Beilstein CrossFire database with stereochemistry unspecified centers even though all the chiral centers are determined in the original structures. Because of the ambiguity of the graphic representation of three-dimensional structures, an R or S symbol according to the Cahn-Ingold-Prelog rule [12] must sometimes be written next to a chiral center. One obvious example is the chiral center surrounded by four other chiral centers.
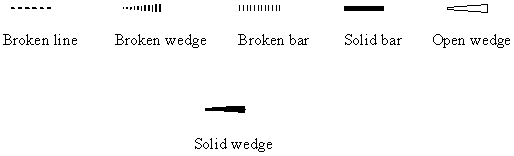
FIGURE 1. Various bonds used for perspective specification.
The confusing diversity of customs is illustrated by the use of broken wedges to represent stereocenters (Figure 2). Many authors use the perspective-misleading formula I [11] whereas others [13] use formula II (Figure 2).
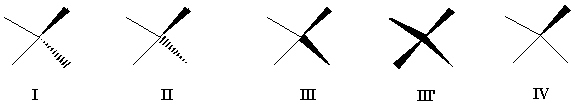
FIGURE 2. Two ways (I and II) of solid wedge and broken wedge representation most frequently seen in the literature. A first proposal [1] advocated the use of only the solid wedge (III or III'). Here, we show that a single solid wedge is sufficient to represent configuration at quadrivalent chiral centers (IV).
In 1996, IUPAC published recommendations for graphic representation of three-dimensional structures [14]. The recommendations for tetrahedral centers are shown in Figure 3. However, this recommendation has received little attention by organic chemists and software companies. The confusing situation persists, even in all organic chemistry textbooks. Thus, a recent book [15] uses a solid wedge and a broken wedge to represent a three-dimensional chemical structure, whereas another [16] uses a solid wedge and a broken bar. In the latest edition of yet another textbook [17], solid wedges and broken lines are used. Clearly, there is a regrettable and confusing lack of agreement.
V VI VII VIII IX
V' VI' VII' VIII' IX'FIGURE 3. Stereochemical representations V - IX recommended by the IUPAC and V' - IX' according to the one-wedge convention introduced in this paper.
Previously, Lin [1] in the first proposal argued that the broken wedge, broken line, solid bar, and broken bar are either not reasonable or can all be replaced by the solid wedge in stereochemical representations of quadrivalent centers. A one-wedge convention of stereochemical representation was therefore proposed. In the original proposal [1], a restriction was made on the relative lengths of the bonds and the arrangements of these bonds. It is now recognized that this restriction is unnecessary.
If two wedges of only one type are used, in agreement with the IUPAC
recommendations, we may use two solid wedges (e.g., formulas III
or III' in Figure 2). The use of several solid wedges may be necessary
to specify many other stereochemical structures, except quadrivalent centers
(e.g., a carbon center), where only one wedge is enough.
Proposal for a One-Wedge Convention
We first argue that one kind of wedge is enough, and the solid wedge
is preferred by far (Figure 2, structure III). This means that a
solid wedge pointing downward from the center can replace the broken bar
in the IUPAC recommendation (Figure 3). Furthermore, a convention based
on the one-wedge symbol is proposed, with a or b replacing
c,
d,
e,
f,
g,
or h (Figure 4).
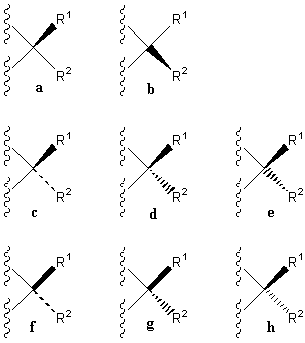
FIGURE 4. The one-wedge symbolization where a or b is
used instead of c, d, e, f, g, or
h, etc.
The proposed convention for quadrivalent centers can be expressed as follows:
It is easy to convert all the structures in Figure 3 (IUPAC recommendation)
to the one-wedge system formulas (V'-IX'). The first step
is to change all the broken bars to the wedges pointing down. The second
step is to keep only one wedge. There is no preference to the up-wedge
(Fig. 5a) or the down wedge (Fig. 5b). Any one of the four wedges in the
full perspective drawings can be used.
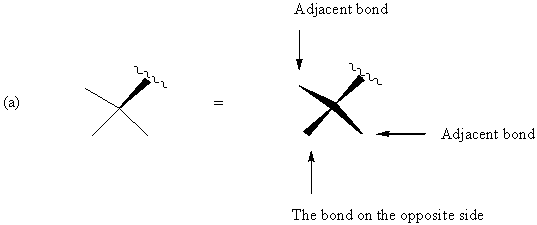
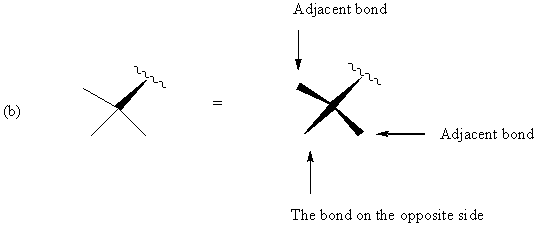
FIGURE 5. Representations of a chiral quadrivalent center in the one-wedge convention and their full perspective drawings. Adjacent and opposite are relative to the way the structures are drawn.
Figure 5 shows that, if we direct attention to a bond adjacent to the original wedge and want to make it the solid wedge, we simply change the direction of the wedge (If the original wedge points upward from the center as seen in Figure 5a, the adjacent bond, if used to indicate the stereochemistry, should have the thick end at the center). If we want the bond on the opposite side of the chiral center to be the only solid wedge, the relation with the center should be unchanged, i.e., the thick end is away from the center in Figure 5a, and the thick end is at the center in Figure 5b. This change will not influence the configuration. Therefore, any single one of the four wedges in the drawings on the right side in Figure 5 can be taken as the stereochemical indicator.
Following item 2, an eclipsed bond can easily be recovered (Figure 6). This follows the accepted perspective representation in the literature, except that only one kind of symbol, i.e. the solid wedge, is used.
It should be noted that the eclipsed ligand can be placed as an adjacent
ligand to the left or to the right of the wedge, it makes no difference.
The revisualized fourth ligand must not be placed opposite to the wedge.
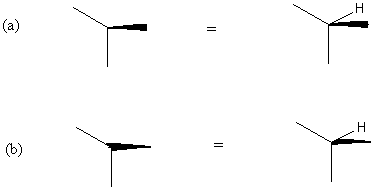
FIGURE 6. Eclipsed bond (only three bonds drawn). Normally, the missing ligand is an electron lone pair or a hydrogen. In (a) the cone bearing the three normal bonds, including the C- H bond, has the opening downward and the cone vertex at the stereochemical center. In (b) the cone bearing the three normal bonds, including the C- H bond, has the opening facing upward and the cone vertex at the stereochemical center.
In some molecules (see below for an example), a chiral quadrivalent
center is surrounded by four other chiral centers. If wedged bonds are
used for stereochemical specification at the center, we need the configuration-specifying
bond to connect the two chiral centers. If a solid wedge connects two chiral
centers, drawing as in Figure 7 is recommended. It should be noted that
the wedge connecting two quadrivalent centers defines the configurations
of the two centers because the wedge connects two cone vertexes.
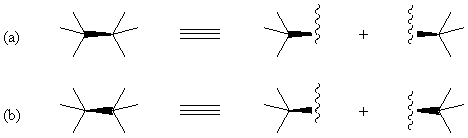
FIGURE 7. The solid wedge allows to describe simultaneously the configuration
of two adjacent chiral centers. The solid wedge connects two cone vertexes.
The relative lengths and the bond distribution of the three normal bonds are immaterial provided that the anticlock order of R1 R2 R3 is the same. For example, 1, 3 and 4 are the same configuration (Figure 8). The wedge can be placed in between any two of the three normal bonds provided that the anticlock order of R1 R2 R3 is the same. For example, 2a, 2b and 2c are the same configuration (Figure 8).
![]()
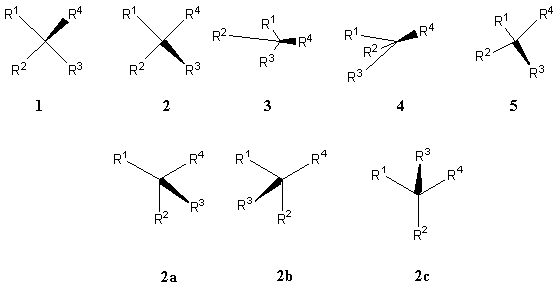
FIGURE 8. Acceptable one-wedge stereochemical representations of an
identical absolute configuration.
In the original proposal [1], we used a triangle defined by the three
ligands. However, it now appears clear that this triangle definition is
not necessary. The definition of a plane including the chiral center [18]
is also unnecessary.
Two Ways of Re-visualization
There are two ways of revisualizing the 3D structures at a chiral center. The first is to follow item 2 of the convention directly. We may first imagine that the three normal bonds are of the same length (4a) and they are uniformly spread to have 120° in between any two bonds (e.g., 4b in Figure 9), so that the mouth of the cone is easily found up (5b) or down (4b).
For experienced chemists, the mouth of the cone defined by the three non-wedge bonds can also be directly envisaged to point to the side opposite to the wedge. If the wedge is up (Figure 5a), the middle normal bond, i.e., the bond depicted in 2D as being opposite to the wedge, is more proximal to the viewer than the center. If the wedge is up (Figure 5a), the bonds depicted in 2D as being adjacent to the wedge is more distal to the viewer than the center.
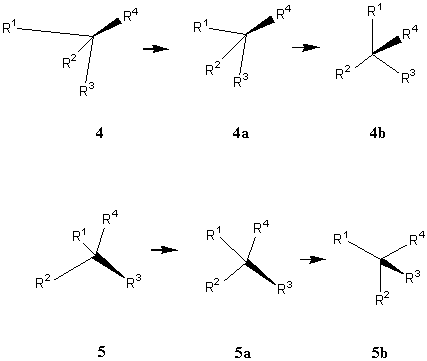
FIGURE 9. Two steps to revisualize the three normal bonds distributed on a cone opposite to the wedge.
The second way is that, when needed to help visualization, all four bonds at a quadrivalent center may be imagined as or actually drawn as wedges, two opposite pointing upward, and the two others downward (Fig. 5a and b). The second method should be applied only to individual centers in an imaginary drawing, because, for a molecule containing contiguously adjacent chiral centers, displaying all the bonds as wedged at one chiral center may have conflicts with other chiral centers.
We prefer and recommend the first method or the so-called cone-method.
We do not recommend that all the four bonds are actually drawn
as wedges if the second method is used.
Advantages of the One-Wedge Convention
The one-wedge convention is correct as far as perspective is concerned, and it is easy to understand, as shown in Figures 5 and 6. Additional advantages exist as discussed below, not to mention that it is also an esthetically appealing representation.
Versatility
All the formulas (1-5 in Figure 8) represent the same configuration. In the original proposal [1], representation 4 was not acceptable. Now representation 4 is found acceptable and has the same configuration as 1-3 and 5. Therefore, when drawing crowded structures such as dendrimers or supramolecular assemblies, some bonds can be arranged very close to each other (e.g., 3), and some bonds may even be longer than others (e.g., 4), yet they all still accurately represent the same absolute configuration if the orientation of the solid wedge is the same and if the three normal bonds are distributed in the same sense. This versatility can also be helpful when using the CIP rules [12] (vide infra).
Simplicity
In many structures, two or more symbols are used for stereochemical representation (Figure 3). In contrast, the one-wedge system uses only one solid wedge per quadrivalent center.
By using only one solid wedge, other bond symbols can be reserved for other purposes of unambiguous indication. For example, instead of using the broken line for stereochemical representation, it can be used to indicate hydrogen bonds and perhaps other weak bonds. A solid bar can be used in other types of stereochemical representation when necessary. For example, solid bar and broken bar can be used for the chirality of a helical, propeller-shaped or screw-shaped molecular entity. With the well-defined one-wedge convention, as shown in Figure 9, even the application of a solid bar (6) is not necessary. Two wedges are readily sufficient (7).
![]()
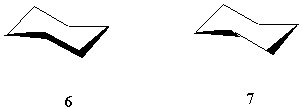
FIGURE 10. The use of a solid bar for perspective drawing is not necessary.
Because any unnecessary complication may cause confusion, simplicity will provide unambiguity. Below, we discuss additional cases showing the clarity of the one-wedge convention.
Lack of ambiguity in the presence of multiple chiral centers
The simplicity of the one-wedge convention can be extended to the cases where a wedge is used to connect two stereochemical centers (Figure 7). The two centers will be simultaneously specified by one wedge (Figure 7). Normally, if possible, the solid wedge will always be placed between a chiral and a non-chiral atom. In the one-wedge convention, however, a wedge can be used to connect two stereochemical centers. The traditional system can not do this since the solid wedge and broken wedge are used to define only one center! A broken wedge connecting two stereochemical centers will automatically produce ambiguity.
Similarly, broken lines and bars as used in stereochemical representation should be banned because they cannot tell which end is closer to the viewer. Clearly, both ends are equally close to the viewer if a solid bar is used as shown in Figure 10. Therefore, the one-wedge system economically, simply and unambiguously uses the solid wedge, and at the same time offers the possibility of the unambiguous and exclusive application of other symbols for other indications such as H-bonds or other weak intermolecular or intramolecular interactions.
There are cases [19-21] where a chiral quadrivalent center is surrounded by four other chiral, quadrivalent centers (e.g., formula 8 in Figure 11). It has been recommended [19-21] that the R or S stereochemistry must be specified at this atom and that no wedge should be used.
Note first that only solid wedges can be used (9). If only solid wedge is used it is possible to use four wedges to give a full perspective drawing at the molecular center in 10. Applying two solid wedges and two broken bars, as required according to the IUPAC recommendation, to the molecular center in this molecule is not satisfactory because all these four bonds connect two chiral centers. Any one of the four bonds at the molecular center in 10 can be drawn as the solid wedge and directly used to unambiguously indicate the configuration, simply by changing the C- H bond at the other end of the wedge at the periphery to a normal bond (11). Applying the one-wedge convention yields 11 and 12.
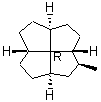 8
8 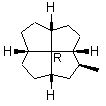 9
9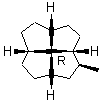 10
10
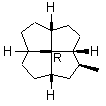 11
11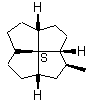 12
12
FIGURE 11. A chiral quadrivalent center is surrounded by four quadrivalent centers.
Constructing the full perspective dawning with four wedges (e.g., 12b in Figure 12) on the central chiral center in 12 is also possible by shifting some bonds (here the three C-H bonds are shifted inside the rings) if only solid wedge is used. Attempted application of two solid wedges and two broken bars, as required according to the IUPAC recommendation, to the central carbon in 12 also requires the shift of these three C-H bonds. The single wedges of the three hydrogen ligands and the eclipsed hydrogen can be placed in such a manner (12a) that if one draws all four wedges at each chiral center the wedges would obey the requirements illustrated in Figs. 5 and 7, and there will be no conflict between two adjacent chiral centers concerning the direction of the wedge they share. For 12b in Figure 12, we shift the four H-C bonds inside the five-membered rings (12a) in order to keep the whole molecular configuration the same as that of 12 and have the same stereochemical centers on the periphery cycle as those of 11. Of course the structures 12 and 12c are identical (In 12c the peripheral C-H bonds are placed in side the rings. This might be difficult if the substituents are a bulk group). The drawing of the obviously better structure 12 is possible only if the one-wedge convention is applied. The eclipsed ligand in 12 should be placed as an adjacent ligand to the left or to the right of the wedge (Figure 6). The revisualized fourth ligand C-H must not be placed opposite to the wedge.
As stated before, the wedge connecting two quadrivalent centers defines the configurations of these two centers because it connects two cone vertexes with three normal bonds distributed on each cone. In a molecule containing contiguously adjacent chiral centers (e.g., 11 and 12), specifying one wedge at one center will not define the configurations at all the other adjacent centers.
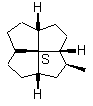 12
12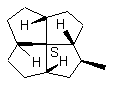 12a
12a 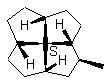 12b
12b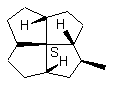 12c
12c
FIGURE 12. It is possible that a chiral quadrivalent center is perspectively fully drawn by using one type of wedge, even though it may be surrounded by four other chiral centers.
Figures 11 and 12 may have showed the advantage of the cone-method (Item 2 of the convention) of revisualization. The sense of the rotation of the three normal bonds determines the configuration. These three bonds are distributed on a cone with the vertex at the considered chiral center. Any possible ambiguity disappears.
Thus, we are able to build a graphic representation of three-dimensional structures even where the previous convention failed and was ambiguous [19-21]. Another example is spirocaracolitone B where the ambiguity problems can be solved by the one-wedge convention (vide infra).
Unambiguous R-S specification by automatic naming
The existence of several stereodrawing conventions produces conflicts
between user specifications and computer interpretations of configuration.
For example, the IUPAC names generated by automatic naming softwares (three
existing naming products, ACD/Name and Index Name, Beilstein AutoNom and
ChemInnovation Nomenclator) are listed below.
|
|
|
|
|
 |
(2S)-2-chlorobutane | (S)-2-Chloro-butane | (2S)-2-chlorobutane |
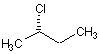 |
(2R)-2-chlorobutane | 2-Chloro-butane | (2R)-2-chlorobutane |
AutoNom of Beilstein Informationssysteme GmbH, Frankfurt, Germany (http://www.beilstein.com).
Nomenclator of ChemInnovation Software, Inc., San Diego, CA, USA (http://www.cheminnovation.com/)
** ACD/Name allows to switch to undirected bonds and an equal name (2S)-2-chlorobutane for both structures will be generated.
*** AutoNom (http://ChemWeb.com/autonom)[22] takes into account only wedges with narrow end at the stereo center.
As an illustration of ACD naming according to the one-wedge convention, structure 12 in Figure 11 gave the identical correct IUPAC name: (1S,2aR,4aS,6aS,8aR,8bS)-1-methyldodecahydropentaleno[1,6-cd]pentalene
Easier R-S specification
If only a symbol R or S is given on a stereochemical center (see for example 8 in Figure 11), with four normal bonds and without any perspective indication, it is much more difficult to visualize this center, more difficult to construct a full perspective structure (e.g., 10), and hence more difficult to prove that a correct assignment of configuration was made. As shown in structures 11 and 12, the center with a wedge connected to it immediately tells if the center has an R or S configuration.
Generally the flexibility and unambiguity of the one-wedge convention
are also helpful to visualize other simple chiral centers and define their
R
or S configuration according to the CIP rule [12]. The wedge with
the thick end at the center is the most convenient format (X and
XI)
for CIP nomenclature purposes. If the priority is 1>2>3>4 and ligand 4
is used as the wedged bond, the S or R-configuration can
immediately be specified (Figure 14).
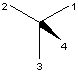 S
S 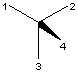 R
R
X XI
FIGURE 14. R and S specification.
An Example
The natural product spirocaracolitone B contains 14 chiral centers and its structure has been elucidated by X-ray crystallography [23]. However, even in the original paper, the stereochemistry of four chiral centers (indicated by * in Figure 15) was not shown. One reason may be the presence of a chiral center surrounded by four chiral centers (the spiro carbon indicated by an arrow in Figure 15). The proposed convention can be applied to this product to draw a structure 13. In the simple case (Figure 15a and b) of a substituent pointing backward, a broken wedge can be replaced by a wedge or by showing the proton pointing upward.
In the last case (Figure 15c), and in order to
avoid the case demanding the configurational bond to be both wedge up,
the H- C bond is explicitly drawn. Therefore,
at one chiral center the wedge is up and at the other center the wedge
is down. The normal H- C bond in Figure 15c
is positioned pointing inside the cyclohexane ring. It should be mentioned
that both quadrivalent centers requiring wedge up can be avoided.
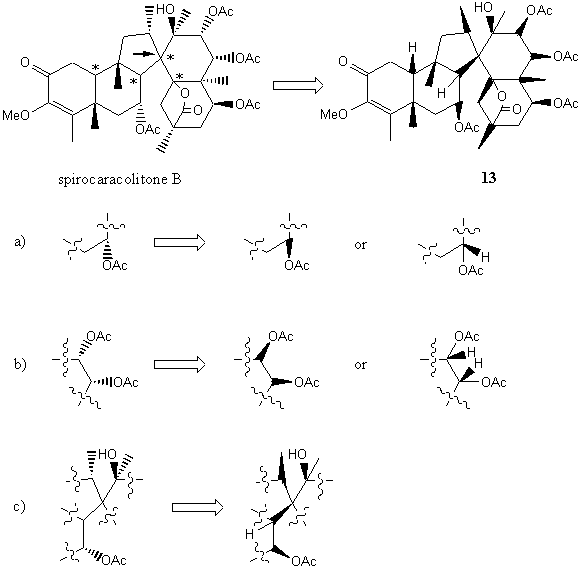
FIGURE 15. An application of the one-wedge
convention to Spirocaracolitones B.
Concluding Remarks
It is broadly believed by many chemists (at the beginning the present authors were also among them!) that a structure drawn in the most popular way (XII in Figure 16) is more appealing and pleasant than the one-wedge representation (XIII or XIV). Why is this so? Because structure XII has some local mirror symmetry with both thick ends at R3 and R4 placed identically away from the center. While it is clear that "what is appealing" is a subjective issue, here we would like to insist that the scientific unambiguity and simplicity must be overwhelmingly more significant than any possible, artificial beauty. Some chemists may not like the solid wedge replacement of broken wedge because of some visual, aesthetic reason. It should be reasonable to represent the asymmetry not only unequivocally but also as apparently as possible without masking the existing asymmetry at the relevant center or centers.
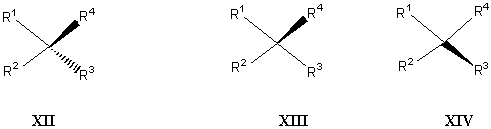
FIGURE 16. Both simplicity and the unambiguity are the beauty of one-wedge
convention.
Chemists are highly interested in asymmetric synthesis and consequently in a convention to represent chiral molecules. However, this should not lead us to cheat by building a perspectively absurd structure XII to create certain false mirror symmetry in structure XII. The one-wedge convention proposed here avoids this trap and offers unambiguity and esthetic appeal.
(We have submitted this proposal to IUPAC for consideration and discussion. A website at http://www.mdpi.org/molecules/wedge.htm has been created for further discussions.)
Acknowledgements: The referees kindly brought references 6, 7 and 8 to our attention. We are grateful to one of the referees for very useful comments.
References and Notes