http://www.chemistrymag.org/cji/2000/025025pe.htm |
May14, 2000 Vol.2 No.5 P.25 Copyright |
The preparation and structure determination of perchlorate tricarbohydrazide manganese (II)
Zhang Tonglai,
Zhang Jianguo, Zhang Zhigang, Wei Zhaorong, Yu Kaibei#
(Department of Mechano-electric Engineering, Beijing Institute of Technology,
Beijing 100081; #Analysis and Measurement Center, Chengdu Branch of the Chinese
Academy of Science, Chengdu 610041, China)
Received Feb. 20, 2000.
Abstract A new coordination compound was
prepared by mixing aqueous solution of carbohydrazide and manganese perchlorate. It was
also characterized by elemental analysis, IR and DSC analysis. The molecular and crystal
structure of perchlorate tricarbohydrazide Manganese (II) was
determined by X-ray crystallography. The crystalline is monoclinic with space group P21/n.
The coordination compound could be expressed in the formula of [Mn(CHZ)3](ClO4)2
with crystal parameters: a = 1.0197(2)nm, b = 0.8593(1)nm, c = 2.1412(3)nm, b= 100.86, V = 1.8426(5)nm3,
Z = 4. The results suggest that all carbohydrazide molecules coordinated with central ion
as bidentates through oxygen atom of carbonyl group and one terminal nitrogen atom, and
linked with Manganese to form three five-number rings.
Keywords manganese perchlorate, carbohydrazide, preparation, molecular structure,
crystal structure
Carbohydrazide is an azotic ligand with lone electron pairs. It may coordinate with many metal ions as monodentate or multidentate [1,2]. Because the ramification of hydrazine possesses strong reduction ability, it has been used to design a variety of compounds with explosive properties. This kind of coordination compounds of carbohydrazide arouses great interests especially in recent years in primary explosives, propellants and high explosives [3-6]. As a part of our research work, we now present the preparation method and the structure determination of perchlorate tricarbohydrazide Manganese (II).
1 EXPERIMENTAL1.1 Preparation
All materials and reagents used in our experiments are reagent grade. The carbohydrazide was commercial product and recrystallized in distilled water. The product is white sheet crystal with a melting point of 155°C.
2.97g of carbohydrazide was dissolved in 20ml distilled water. The solution was put into a reactor and heated to 60-70°C in water bath. The solution of manganese perchlorate was obtained by reacting manganese carbonate (1.17g, 0.01mol) and equivalent perchlorate acid [5,6]. Its filtrate was then dropped into the reactor with violent stirring and allowed to react for 35 mins. The reaction mixture was then cooled to room temperature and filtered to obtain white crystal product, which was washed with distilled water and dried in vacuum oven at 60°C for 2 hrs. The yield was 76%, M.P. 265-266.3°C (d). The product is a potential strong explosive material and must be treated with great care.
Controlled evaporation process was used to culture single crystals within a few days. The content of carbon, nitrogen and hydrogen was analyzed by Carlo Erba 1106 automatic micro-analyzer. Found: C, 6.85, H, 3.50, N, 32.07. Calcd.: C, 6.87, H, 3.46, N, 32.08.
1.2 Infrared absorption spectrum analysis
Infrared absorption spectra were recorded on Perkin-Elmer 683 FT-IR spectrometer with KBr tablets. The absorption peaks and assignments are as follows:
1.3 DSC analysis
DSC experiments were carried out on model CDR-1 differential scanning calorimeter with an aluminum sample pan. The conditions of DSC were as follows: sample mass about 1 mg, heated rate 10°C/min, static air atmosphere, α-Al2O3 reference sample, Ni/Cr-Ni/Si thermal-couple.
The decomposition processes of the title compound were recorded in the range of 30-500°C under linear temperature increase condition. The measurement results showed that the thermal decomposition processes comprise three major stages on the DSC curve. The first stage was a weakly endothermic peak, resulting from melting process of the compound from 263.3°C to 268.7°C. The second stage was an acute exothermic process and showed an acute and strong pinnacle from 268.7°C to 295.6 with maximal at 276.7°C. The third stage showed an overlapped exothermic process from 298.8°C to 330.9°C, and the two peaks appear at 309.8°C and 315.6°C. The DSC results verified that the decomposition process is a very strong exothermic reaction similar to other explosives.
1.4 Crystal structure determination
A single crystal with dimensions of 0.50*0.48*0.28 mm3 was used to collect the diffraction intensity data on a Siemens P4 diffractometer fitted with graphite-monochromated Mo Ka radiation, l=0.071073nm. The measurement was carried out at room temperature. The w scan method was employed and a total of 4247 reflections were collected in the range of 1.94°<q<26.00°. The scan range is: 0< h <12, 0< k <10 and 26 <l <25, in which 3631 independent reflections were obtained, among which 2789 with I>2σ(I) were used for the determination of crystal structure and refinement. The crystal of [Mn(CHZ)3](ClO4)2 is monoclinic with space group P21/n with a = 1.0197(1)nm, b = 0.8593(1) nm, c = 2.1412(1) nm; b=100.86°, V = 1.8426(5) nm3, Z = 4, Dc =1.889 g/cm3, μ=1.089 mm-1, F(000)=1068. Table 1 Experimental data and summary of intensity collection and structure data
| Name | perchlorate tricarbohydrazide Manganese(II) |
Formula |
C3H18Cl2MnN12O11 |
Color |
White |
Mr |
524.13 |
Crystal system |
Monoclinic |
Space group |
P21/n |
Temp. /K |
288(2) |
| Cell constants (2.56°<q<16.47°) | 26 |
Absorption correction (μ)/cm-1 |
1.089 |
Criterion for observed reflections |
I>2 σ(I) |
Data /restraints/parameters |
3627/0/327 |
Goodness-of-fit on F2 |
1.060 |
Final R indices [I>2 σ(I)] |
R1=0.0488, wR2=0.1393 |
R indices (all data) |
R1=0.0624, wR2=0.1517 |
Max. And min. transmission |
0.9787 and 0.7923 |
Refinement method |
Full-matrix least-squares on F2 |
Extinction coefficient |
0.0117(14) |
Largest diff. Peak and hole (e nm-3) |
943 and 672 |
| Atom | X/a |
Y/b |
Z/c |
* Ueq |
Mn |
3817(7) |
1481(6) |
1182(2) |
3.1(2) |
Cl1 |
890(9) |
1303(1) |
2547(4) |
3.9(3) |
Cl2 |
9304(1) |
6687(3) |
923(5) |
5.1(3) |
O1 |
5616(2) |
65(3) |
1432(1) |
3.5(6) |
O2 |
5110(3) |
3367(3) |
1053(4) |
4.1(6) |
O3 |
3616(2) |
661(3) |
232(1) |
3.7(6) |
O4 |
1009(4) |
2021(5) |
1968(2) |
7.7(1) |
O5 |
1326(5) |
2356(5) |
3046(2) |
9.8(4) |
O6 |
1687(4) |
-83(4) |
2632(2) |
7.4(10) |
O7 |
-434(4) |
935(7) |
2555(3) |
11.5(2) |
O8 |
10386(9) |
5932(4) |
1202(3) |
25.9(6) |
O9 |
8295(10) |
5788(7) |
857(6) |
28.4(7) |
O10 |
9397(5) |
7293(6) |
330(2) |
10.5(2) |
O11 |
9161(1) |
7880(8) |
1349(3) |
19.0(4) |
N1 |
4235(4) |
1230(5) |
2264(2) |
4.3(8) |
N2 |
5463(3) |
454(4) |
2459(2) |
4.2(8) |
N3 |
7106(3) |
-1065(4) |
2203(2) |
4.4(8) |
N4 |
7500(4) |
-1393(6) |
2853(2) |
5.8(1) |
N5 |
2479(3) |
3629(4) |
955(2) |
4.0(8) |
N6 |
3254(3) |
4829(4) |
765(2) |
4.3(8) |
N7 |
5310(4) |
5826(4) |
736(2) |
4.5(8) |
N8 |
4693(5) |
7261(4) |
548(2) |
5.4(1) |
N9 |
1992(4) |
-154(5) |
1015(2) |
4.5(9) |
N10 |
1771(4) |
-658(5) |
381(2) |
5.0(9) |
N11 |
2507(4) |
-791(4) |
-565(5) |
4.4(8) |
N12 |
1433(5) |
-1772(7) |
-802(2) |
8.8(2) |
C1 |
6035(3) |
-187(4) |
2011(2) |
3.0(7) |
C2 |
4570(4) |
4633(4) |
862(2) |
3.3(8) |
C3 |
2668(4) |
-220(4) |
22(2) |
3.3(8) |
*
| bond | length/nm |
bond |
length/nm |
bond |
length/nm |
Mn -O3 |
0.2125(2) |
Cl2 -O9 |
0.1273(8) |
N3 -N4 |
0.1401(5) |
Mn -O2 |
0.2141(3) |
Cl2 -O8 |
0.1320(5) |
N5 -N6 |
0.1405(5) |
Mn -O1 |
0.2182(3) |
Cl2 -O10 |
0.1392(4) |
N6 -C2 |
0.1330(5) |
Mn -N1 |
0.2286(3) |
Cl2 -O11 |
0.1398(6) |
N7 -C2 |
0.1330(5) |
Mn -N5 |
0.2293(3) |
O1 -C1 |
0.1253(4) |
N7 -N8 |
0.1409(5) |
Mn -N9 |
0.2305(4) |
O2 -C2 |
0.1253(4) |
N9 -N10 |
0.1402(5) |
Cl1 -O7 |
0.1390(4) |
O3 -C3 |
0.1243(4) |
N10 -C3 |
0.1355(5) |
Cl1 -O5 |
0.1407(4) |
N1 -N2 |
0.1411(5) |
N11 -C3 |
0.1331(5) |
Cl1 -O4 |
0.1410(3) |
N2 -C1 |
0.1331(5) |
N11 -N12 |
0.1399(5) |
Cl1 -O6 |
0.1433(3) |
N3 -C1 |
0.1327(5) |
The structure was solved by direct method and refined by full-matrix least-squares methods based on F with anisotropic displacement parameters for the non-H atoms. The positions of the H atoms were found in differential Fourier maps and refined using isotropic displacement parameters. A semi-empirical extinction correction was applied to Fc. The refinement continued to final R=0.0488 and Rw=0.1393 for 3631 independent reflections. The structure solution and refinement are accomplished by using the Siemens SHELXTL 5.03 programs package with the Eclipes/140 computer. Crystal data are listed in Table 1. Fractional atomic coordinates and thermal parameters of [Mn (CHZ)3](ClO4)2 are given in Table 2. Selected bond lengths and angles are provided in Table 3 and Table 4.
2 RESULTS AND DISCUSSIONS
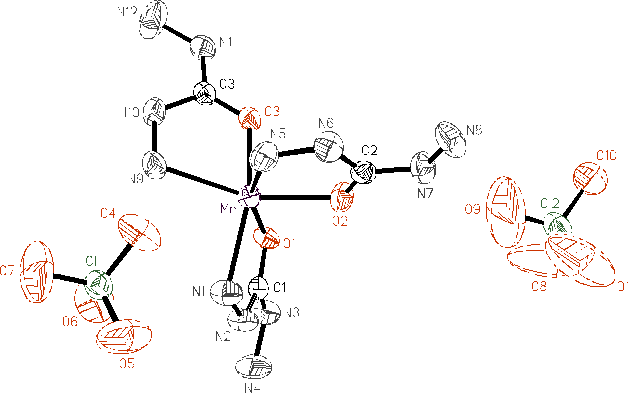
The molecular structure of perchlorate tricarbohydrazide manganese (II) and the atom
labeling scheme are illustrated in Figure 1. The results show that there are three
carbohydrazide molecules in every molecule. Every carbohydrazide acts as a bidentate and
coordinated to manganese cation with its oxygen atom of the carbonyl group and the
terminal nitrogen atom so that they formed a chelating ring with five members. There are
three such rings in the molecule. The coordination number of the central atom is six with
a octahedral configuration. The atoms forming every five-member ring take on the favorable
coplanarity.
| bond | Angle/° |
bond |
Angle/° |
O3 -Mn-O2 |
94.45(1) |
O9 -Cl2-O11 |
109.6(8) |
O3 -Mn-O1 |
88.55(1) |
O8 -Cl2-O11 |
104.3(5) |
O2 -Mn-O1 |
86.76(1) |
O10 -Cl2-O11 |
110.7(4) |
O3 -Mn-N1 |
154.79(1) |
C1 -O1-Mn |
116.9(2) |
O2 -Mn-N1 |
101.57(1) |
C2 -O2-Mn |
117.1(2) |
O1 -Mn-N1 |
73.23(1) |
C3 -O3-Mn |
118.4(2) |
O3 -Mn-N5 |
96.78(1) |
N2 -N1-Mn |
109.3(2) |
O2 -Mn-N5 |
73.71(1) |
C1 -N2-N1 |
117.9(3) |
O1 -Mn-N5 |
160.06(1) |
C1 -N3-N4 |
119.1(3) |
N1 -Mn-N5 |
106.29(1) |
N6 -N5-Mn |
107.9(2) |
O3 -Mn-N9 |
73.77(1) |
C2 -N6-N5 |
117.9(3) |
O2 -Mn-N9 |
160.17(1) |
C2 -N7-N8 |
119.5(4) |
O1 -Mn-N9 |
108.37(2) |
N10 -N9-Mn |
108.4(2) |
N1 -Mn-N9 |
95.20(1) |
C3 -N10-N9 |
117.4(3) |
N5 -Mn-N9 |
91.6(2) |
C3 -N11-N12 |
120.9(3) |
O7 -Cl1-O5 |
107.7(4) |
O1 -C1-N3 |
120.8(3) |
O7 -Cl1-O4 |
110.8(3) |
O1 -C1-N2 |
121.9(3) |
O5 -Cl1-O4 |
108.6(3) |
N3 -C1-N2 |
117.3(3) |
O7 -Cl1-O6 |
109.8(3) |
O2 -C2-N6 |
122.0(3) |
O5 -Cl1-O6 |
110.3(3) |
O2 -C2-N7 |
120.2(4) |
O4 -Cl1-O6 |
109.5(2) |
N6 -C2-N7 |
117.7(3) |
O9 -Cl2-O8 |
109.9(9) |
O3 -C3-N11 |
121.0(3) |
O9 -Cl2-O10 |
108.5(5) |
O3 -C3-N10 |
121.8(3) |
O8 -Cl2-O10 |
113.7(5) |
N11 -C3-N10 |
117.2(3) |
Due to these three chelate-rings, the molecular
structure of [Mn(CHZ)3](ClO4)2 possesses particular
stability. Because every carbohydrazide molecules has one free diazanyl in the molecular
structure, they served as spring between molecules that makes the molecular structure
possess good flexibility of which is accordant with its lower mechanical sensitivity[7].
The outer spheres are two perchlorate anions. They combined to inner sphere by static
electricity.
3 CONCLUSION
The obtained results show that the coordination compound is expressed as [Mn(CHZ)3](ClO4)2
. All of the carbohydrazide molecules serve as bidentates to chelate with manganese
cation forming three five-member rings, which let the coordination compound possess very
stable structure. The higher nitrogen content of the ligand and stronger oxidization
ability of perchlorate make the compound give out high energy output when it is ignited.
Consequently, perchlorate tricarbohydrazide manganese (II) would be a kind of prominent
and potential energetic material for mining applications.
REFERENCES
[1] Dutta R L. Innorg. Nucl. Chem., 1981, 43 (10): 2557-2562.
[2] Bansho T. JP 04,343,359, 1992.
[3] Fogelzang A E. Mater. Res. Symp. Proc., 1996, 418-422.
[4] Zhang T L, Lu C H, Qiao X J et al. Chinese J. Struct. Chem. (Jiegou Huaxue),
1999, 18 (6): 432-436.
[5] Lu C H, Zhang T L, Wei Z R et al. Chinese Journal of Inorganic Chemistry
(Wuji Huaxue), 1999, 15 (3): 377-383.
[6] Wei Z R, Zhang T L, Lu C H et al. Chinese Journal of Inorganic Chemistry
(Wuji Huaxue), 1999, 15 (4): 482-486.
[7] Lu C H, Zhang T L, Wei Z R et al. Proceedings of the third International
Autumn Seminar on Propellants, Explosives and Pyrotechnics Sichuan Publishing House of
Science of Technology, Chengdu, 1999, 33-36.