http://www.chemistrymag.org/cji/2002/041005ne.htm |
Jan. 1,
2002 Vol.4 No.1 P.5 Copyright |
(Institute of Military Medicine, Headquarters of General Equipment, Beijing 100101; #Academy of Military Medical Sciences, Beijing 100850)
Received on August 7, 2001
Abstract A novel multiple-channel instrument is developed which is designed for detecting hydrazine compounds detection of 10-6 level in real time. The instrument employs electrochemical sensors which have the advantage of high specificity and fine stability. It can be used for leakage checking in the places where a large quantities of hydrazine are used or manufactured.Keywords Hypergolic vapor, Electrochemical sensor, Vapor detection 1 INTRODUCTION
Large quantities of hypergolic propellants including hydrazine(HZ), monomethy hydrazine(MMH), and unsymmetrical-dimethylhydrazine (UDMH) are used extensively as rocket fuels in the national defence industry. These three hydrazine compounds are toxic, combustible and explosive. Some research indicated that the hydrazine compounds are even carcinogenic. The current recommended exposure level(TLV) are 0.1, 0.04, 0.5 ppm in air for HZ, MMH, and UDMH, respectively.
Methods for determination of these three hydrazine(HZ,MMH,UDMH) include the earlier methods of colorimetry, titration with standard potassium iodate solution and sophisticated instrumental analyses involving gas chromatography, mass spectrometry, spectroscopy, photo-ionization, semi-conductors, chemiluminescence and electrochemistry. All of these methods except the electrochemistry suffer from the drawbacks such as complexity,insufficient sensitivity and lack of specificity, continuous measurement capability, or the cost-effectiveness necessary to meet present requirement. In addition, there is still no effective domestic instrument for determination of hypergolic vapor in real-time. Imported instrument for hypergolic vapor detection has the limitations of either expensive or inadequate to be adapted for actual applications.
In this work, a new mutiple-channel monitoring instrument for propellant of hydrazine compounds has been developed. Electrochemical sensor has been employed which can provide fast, sensitive and real-time determination of HZ,MMH and UDMH vapors at 10-6 levels when coupled with an instrument system. 2 INSTRUMENT DESIGN AND CHARACTERIZATIONS
There are four primary sections for the design of the instrument, including the design of electrochemical cell, the hydrazines dilution system, the intellectual electrochemical sensor and the mail processor system. The following report discusses the development of the four sections.
2.1 The development of electrochemical cell
The fundamental design of the instrument is the electrochemical cell with a three-electrode system.A schematic diagram of the electrochemical sensor design is shown in Figure 1.The three electrodes are all Teflon-bonded diffusion electrodes which are prepared by spraying the catalyst-Teflon dispersion onto the Teflon film.The catalyst materials are purchased as high purity powder of gold and platinum catalyst. The electrodes are sealed inside a polyethylene chamber which is filled with certain alkaline electrolyte.The alkaline electrolyte is prepared from reagent grade materials and distilled water.The chamber is assembled with special technology to ensure the cell impermeable.

Fig 1 The structure of the diffusion electrochemical cell The gold and platinum leads of the sensor electrodes are connected to a potentiostat which is capable of variable bias and this is required for optimum signal stability.
During the operation of the sensor, mixtures of hydrazines in nitrogen are passed over the sensing electrode at constant flow rate. The electrochemical reaction occurs.The oxidation-reduction reactions of the three hydrazine compounds on the sensing electrode are as follows:
N2H4+4OH-
CH3NHNH2+4OH-
(CH3)2NNH2+2OH-
The current produced by electrochemical reaction in the sensor between the sensing and counter electrode is measured and this is directly proportional to the concentration of hydrazine vapors. So the concentrations of the hydrazine vapor are determined.
The main parameters of the hydrazine electrochemical cell are illustrated as follows:
The concentration ranges:0-100ppm; minimum detectable sensitivity: 0.2ppm ; zero drift: <±5% of full scales per week; accuracy: 10% F.S. (when the concentration of hydrazines is 50ppm); response time: 90% of signal with 60s; fall time: 90s;operating temperature range: -10-40oC; operating relative humidity range: 25%-85%; average life span: ≥one year.
However, the present limitation of the electrochemical cell are its response time and average lifetime.These characteristics can be improved by further sensor developments and more field-testing .
2.2 The development of dynamic dilution system for hydrazines
Calibration, testing and evaluation of the instrument require some apparatus to generating a stable hydrazine stream in the desired concentration range. However, hydrazine compounds are prone to being decomposed and adsorbed on the container walls. It is difficult to keep such a system properly adjusted because a change at any point in the system will upset the flow at all the other points.
A dynamic hydrazine dilution system is developed to make hydrazine vapor mixtures of 0-100ppm in a continuous manner .The dilution system is contained in a large fume hood,since the hydrazines are toxic and explosive.A syringe which is driven by a syringe pump is used to inject hydrazine compounds into a diluent gas stream flowing at a controlled rate(99.9998% N2 was used as diluent) .The diluent gas passes through a pre-conditioning chamber which is used to warm the gas stream when relatively high concentration(>50ppm) of hydrazines are desired.The diluent passed the syringe needle, picking up the hydrazine vapor and into a container which the hydrazines and N2 are mixed. The vapor mixture then may be divided by two Teflon valves.The vapor mixtures may either be collected in Teflon bags or be used directly from the sample exit stream.
The theoretical concentration of the vapor can be found by knowing the delivery rate of the liquid and the flow rate of the diluent.The equation giving the ppm vapor concentration by volume is:
here : A=the delivery rate of hydrazine liquid(ml/min), D=the density of the liquid (g/ml), M=the molecular weight of the liquid(g/mol), F=the flowrate of the diluent(ml/min).
During calibration procedure,spectrophotometric analyses are used to determine the concentration of hydrazine compounds in the generator output gas stream.The method in GJB2373-95 can be used in calibration procedure.According to the GJB2373-95,the concentration of the hydrazines were determined colorimetrically at a wavelength of 460nm for HZ, 470nm for MMH and 500nm for UDMH. 2.3 The development of intellectural electrochemical sensor
The principle of the intellectual sensor is illustrated in Figure 2.The concentration of hydrazine is converted to electrical signal at mA level after oxidation-reduction reaction in electrochemical cell.The mA signal is amplified to 0-200mV signal which is then converted to digital signal by the analog-digital conversion of the MCU.The digital signal which is calibrated as real-time concentration of hydrazines are shown with
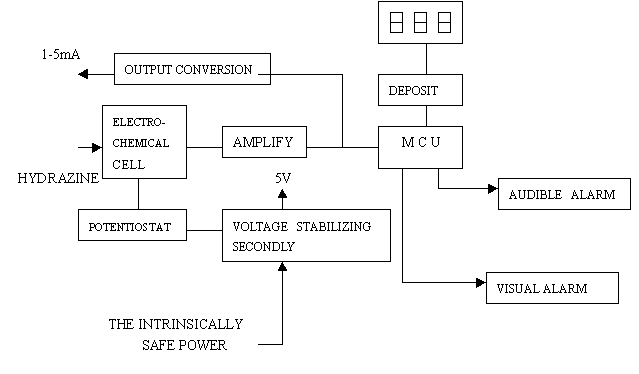
Fig 2 The intellecture sensor
The intellectual
functions of the sensor consist of zero offset automatic adjust, trouble automatic
detection,sensitivity compensation and non-linearity compensation ,which are provided by
the software programme of the MCU in the sensor.The software programme of the intellectual
sensor accommondates five parts including initiating, analog-digital conversion,trouble
judging,alarm and digital display.
Because the sensors are located in the adverse circumstances,the sensor
has other characteristics of impermeable,anticorrosive and antimagnetic with particular
shielded material.
2.4 The development of the main processor
The diagram of the main processor is shown in Fig3.It consists of the MCU and accessory
circuit including the intrinsically safe circuit.18V,180mA of intrinsically safe power is
supplied to the sensor which can be located in the explosive environment. The main
processor is linked with 8 or 16 electrochemical sensors distributed over different work
places.The real-time concentration of every working point can be displayed circularly on
the LED display window of the main processor panel showing the levels in parts per
million(ppm) with ![]() figures.The display is shown for 3 senconds while updated every 3 seconds.Audible and
visual alarm are provided when hazardous situation occurs and the display on the main
processor will pinpoint the alarm area.The voice of the alarm is over 90dB and the
brightness for alarm can be distinguished from 10 meters away. The alarm is set at two
levels,the first level is predictable alarm with the yellow LED lit and the second is the
dangerous alarm with the red LED lit. Daily data of the gas concentration can be stored or
printed by the main processor. What is more, the output data of the main processor can be
sent out to the higher level unit in the form of series out which not only can monitor the
concentrations of the work places, but can control the work of the instrument through
computer networks. This higher level of monitoring and control system is the item we
intend to develop in the coming years.
figures.The display is shown for 3 senconds while updated every 3 seconds.Audible and
visual alarm are provided when hazardous situation occurs and the display on the main
processor will pinpoint the alarm area.The voice of the alarm is over 90dB and the
brightness for alarm can be distinguished from 10 meters away. The alarm is set at two
levels,the first level is predictable alarm with the yellow LED lit and the second is the
dangerous alarm with the red LED lit. Daily data of the gas concentration can be stored or
printed by the main processor. What is more, the output data of the main processor can be
sent out to the higher level unit in the form of series out which not only can monitor the
concentrations of the work places, but can control the work of the instrument through
computer networks. This higher level of monitoring and control system is the item we
intend to develop in the coming years.
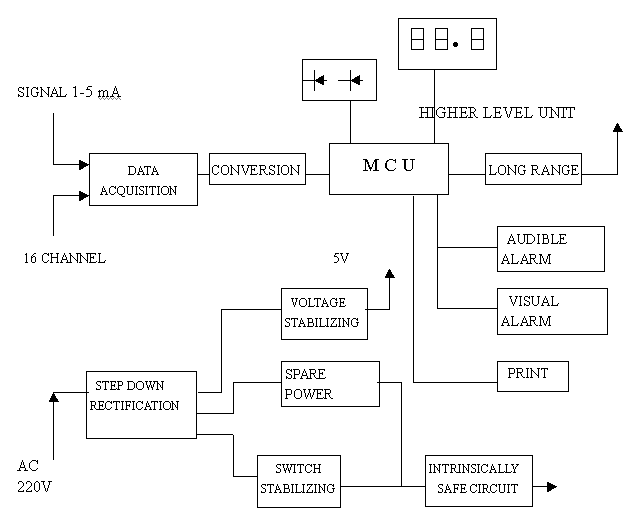
Fig 3 The main processor
The multiple-channel instrument will be installed for leakage checking in places where a large quanties of hydrazine compounds are used or manufactured. It is estimated that the instrument for a hypergolic vapor detection instrument will be put into operation by the end of this year. The fulfilment of this accurate and reliable monitoring instrument system is very imperative for the protection of the environment and nearby personnel.
REFERENCES
[1] John A E. A D A, 094968, 1980.
[2] Saunders R A. A D A, 054636, 1978.
[3] Dan C, Dalee L. N 96-19066, 1996.
[4] Joseph R S. N 77-23439, 1977.
[5] You T Y, Niu L, Gui J Y et al. Pharm. Biomed. Anal., 1999, 19 (1-2): 231-237 .
[6] Zhou W H, Xu L, Wu M J et al. Anal. Chim. Acta, 1994, 299 (2): 189-94.