http://www.chemistrymag.org/cji/2005/071004ne.htm |
Jan.21, 2005 Vol.7 No.1 P.4 Copyright |
Luo Huimou, Li Yiqun
(Department of Chemistry, Jinan University, Guangzhou 510632, China)
Abstract Treatment of aromatic aldehydes
with iodine and ammonia water using room temperature ionic liquids
1-butyl-3-methylimidazolium tetrafluoroborate at room temperature afford the corresponding
nitriles in high yields. The notable advantages of this protocol are such as no need of
catalyst, mild conditions, simple operation, short reaction time, high yields and
recycling of the ionic liquid.
Keywords ionic liquid; aromatic aldehydes; nitriles; iodine; ammonia water
The remarkable synthetic properties of the
nitrile group have ensured long standing studies of their utilization in organic synthesis[1].
There are only a few methods known for the conversion of aldehydes to nitriles in one-pot
procedure[2]. Unfortunately, most of these methods suffer from serious
drawbacks which include use of hazardous (selenium dioxide)[3] and expensive
(hydroxylamine o-sulfonic acid)[4], (o-2,4-dinitrophenylhydroxylamine)[5]
or commercially nonavailable reagents, long reaction time, low yields, drastic reaction
conditions, and tedious workup procedure. Therefore, there is a need for the development
of protocols using readily available and safer reagents which lead to high yields of
nitrile compounds. Recently, NaN3/AlCl3[6], ClSi(N3)3[7],
H2NOH.HCl/phathalic anhydride/Et3N[8], I2/ammonia/THF[9],
Graphite/H2NOH.HCl/MeSO2Cl[10] and H2NOH.HCl/dry
Al2O3/MeSO2Cl[11], etc. have been reported as
the new protocol for this one-pot conversion. Synthetic chemists continue to explore new
methods to carry out the direct synthesis of nitriles from aldehydes.
Room temperature ionic liquids (RTILs) have aroused increasing interest
for their promising role as alternative media in synthesis, separation, and
electrochemistry as a result of their unique chemical and physical properties[12].
RTILs can dissolve a wide spectrum of organic, organometallic, and inorganic compounds.
Also, they have no detectable vapor pressure and are relatively thermal stable. So, there
is no loss of solvent through evaporation with RTILs. This will avoid environmental and
safety problems due to volatilization, as is the case in traditional organic solvents.
Therefore, they are proposed as novel solvent systems to replace traditional solvents that
are generally toxic, flammable, and volatile. RTILs are regarded to have the potential to
be alternative reaction media for "Green chemistry" [13].
We herein report the use of 1-butyl-3-methylimidazolium
tetrafluoroborate ([Bmim]BF4) ionic liquid for the synthesis of nitriles from
aromatic aldehydes with iodine and ammonia water under mild conditions (Scheme 1). And
ionic liquids could be recovered and reused for more than five times without losing any
activity.
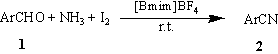
Scheme 1
We found that aromatic aldehydes were readily converted to give the
corresponding nitriles in good yields. The results obtained were compiled in Table 1.
Table 1 Conversion of aldehydes into nitriles using [Bmim]BF4
Entry |
Aldehydes(1) |
Product (2)a |
Time /h |
Yield / %b |
a |
|
|
2 |
89 (85)c |
b |
|
|
2 |
75 |
c |
|
|
2.5 |
76 |
d |
|
|
2.5 |
90 |
e |
|
|
2 |
78 |
f |
|
|
2 |
96 |
g |
|
|
2.5 |
72 |
h |
|
|
2 |
80 |
i |
|
|
2.5 |
90 |
a
Products were characterized by their melting points, IR spectra according to the reported literature, known samples obtained from the commercially available or prepared in current method.b Yields refer to pure isolated products.
c Yield in parenthesis obtained from the ionic liquid in the case of the forth run.
Typical procedure for synthesis of nitriles in the presence of iodine and ammonia water in [Bmim]BF4: Ionic liquid [Bmim]BF4 (5mL), ammonia water (10mL) and aromatic aldehyde (2.5mmol) were previously charged into the 25 ml round-bottom flask equipped with a magnetic stirrer. Under vigorous stirring, iodine (2.75 mmol) was added slowly in portion during 60 minutes. The mixture was stirred vigorously at room temperature for the appropriate time (Table 1). The dark solution became colorless(or light gray in some cases) after stirring for 10-25 min per portion, an indication that the reaction was complete. After completion of the reaction, as indicated by TLC, the reaction mixture was washed with diethyl ether (3×10 mL). The combined ether extracts were concentrated in vacuo after quenched with saturated NaHSO3 solution. The products were confirmed by melt point, IR spectra. The ionic liquid was recovered by extracting the ammonia water phase with dichloromethane and used in subsequent runs after removing dichloromethane.
REFERENCES
[1] (a) Sandler S R. Karo W. Nitriles(cyanides).In organic functional group
preparations, Academic Press Inc., San Diego, 1983, vol.12-I of Organic Chemistry, Chapter
17; (b) Smith R F, Albright J A, Waring A M. J.Org.Chem., 1966, 31: 4100.
[2] Karmarkar S N, Kelkar S L, Wadia M S. Synthesis, 1985, 510.
[3] Sosnovsky G, Krogh J A, Umhoefer S G. Synthesis, 1979, 722.
[4] Fizet C, Streith J. Tetrahedron Lett., 1974, 3187.
[5] Miller M, Loudon G. J.Org.Chem., 1975, 40: 126.
[6] Suzuki H, Nakaya C. Synthesis, 1992, 641.
[7] Elmorsy S S, El-Ahl A S, Soliman H, Amer F A. Tetrahedron Lett., 1995, 15:
2639.
[8] Wang E C, Lin G J. Tetrahedron Lett., 1998, 39: 4047.
[9] Talukdar S, Hsu J L, Chou T C, Fang J M. Tetrahedron Lett., 2001, 42: 1103.
[10] Sharghi H, Sarvari M H. Synthesis, 2003, 243.
[11] Sharghi H, Sarvari M H. Tetrahedron, 2002, 58: 10323.
[12] (a) Wasserscheid P, Keim W. Angew. Chem. Int. Ed., 2000, 39: 3772; (b) Sheldon
R. Chem. Commun., 2001, 2399.
[13] (a)Stephen K P. Chem. Eng. News, 2001, 79: 27; (b) Wasserscheid P, van Hal R, Bosmann
A. Green Chem., 2002, 4: 400.
在离子液体介质中采用芳醛与氨水和碘一锅法直接合成芳腈
罗慧谋,李毅群*
(暨南大学化学系,中国广州510632)
国家自然科学基金(20272018)和广东省自然科学基金(021166,
04010458)资助
摘要 在室温条件下,以室温离子液体1-丁基-3-甲基咪唑四氟硼酸盐为反应介质,采用芳醛与氨水和碘直接反应,高产率合成相应的的腈。该法具有不需催化剂,反应条件温和,操作简单,反应时间短,产率高和离子液体能循环使用的优点。
关键词 离子液体,芳醛,腈,碘,氨水