http://www.chemistrymag.org/cji/2005/078055ne.htm |
Aug. 12, 2005 Vol.7 No.8 P.55 Copyright |
Che Yiping1, 2, DingXiaojing2,
ZhaoShan2, Liu Pengyan1
(1 College of Chemistry and Environmental Science, Hebei University, Baoding
071002, China; 2Beijing Center for Disease Prevention and Control, Beijing
100013, China)
Abstract A new Reversed
Phase High Performance Liquid Chromatographic method (RP-HPLC) has been established for
the rapid determination of polyhexamethylenebiguanide (PHMB) in compound chemical
disinfectants. PHMB is well separated from matrices on a Shiseido C18 (150 ×
4.6mm, 5mm) column with a
temperature at 30ºC, using a mobile phase of A:B = 16:84 (A: acetonitrile, B: 20mmol/L
ammonium acetate, adjusted with acetic acid to pH 4.0) and detected at 235nm. Good
linearity was in the range from 100mg/L to 1000mg/L with relative standard deviation of
1.2% (n = 7). Recoveries at three different levels (100, 200, 400mg/L) were 99.06, 102.4
and 92.92%, respectively. The limit of detection was 50mg/L (S/N = 3). The present method
is simple, rapid and suitable for the routine accurate assay of PHMB in compound chemical
disinfectants.
Keywords RP-HPLC, polyhexamethylenebiguanide, compound chemical
disinfectants
1 INTRODUCTION
Polyhexamethylenebiguanide (PHMB), a newly reported disinfectant in recent years, is a
polymeric compound with low skin irritancy, low eye toxicity, fast speed of kill, stable
and effective over wide pH range (2-11). It is un-soluble in water and usually prepared in
the water-soluble form of hydrochloride salt at a concentration of 20% aqueous solution
(w/v) with a molecular structure as shown in Fig.1.
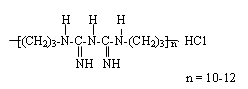
Fig.1 The molecular structure of PHMB
It has been found to be
effective against a wide variety of bacteria and virus at low dose levels even in hard
water. For example, PHMB at a concentration of 0.1% (w/v) can kill AIDS virus within 5
min. Most of all, PHMB is degradable and harmless. Therefore, it has been widely used for
the formulation of compound chemical disinfectants and sanitizers, for use in industrial,
agricultural, food and personal care product (i.e. ophthalmic solutions) disinfection
applications. Its levels vary significantly in different application products, ranging
from high concentrations (0.1% (w/v)) in several compound chemical disinfectants to a low
concentration (0.0001% (w/v)) in ophthalmic solutions. If the concentration of PHMB is
lower than the manufacturer's specification, the disinfectants will not effectively kill
bacteria and virus. In order to supervise the quality of compound chemical disinfectants,
the Ministry of Public Health of China has recommended that the accurate assay of PHMB in
disinfectants is one of the effective methods for quality control purpose and instrumental
methods are recommended for the first choice [1]. Therefore, instrumental
methods with rapidity and simplicity for the accurate assay of PHMB in compound chemical
disinfectants are required for product quality control.
Reversed-Phase High Performance Liquid Chromatography (RP-HPLC)
with Photo Diode Array detector (PDA) is the most frequently used method for routine
determination. However, to the best of our knowledge, no RP-HPLC method for the analysis
of PHMB in disinfectants has been reported. So, the aim of the present work is to
establish a simple and rapid HPLC method for routine assay of PHMB in compound chemical
disinfectants.
2. EXPERIMENTAL
2.1 Reagents and chemicals
The water for the preparation of all solutions was made by a Millipore Milli-QRG
ultra-pure water system (Bedford, MA, USA). The PHMB (20%) standard was supplied by Lvsan
Chemical Ltd. Corp. (Beijing, China). HPLC-grade acetonitrile was purchased from Dima
Technology Inc. (USA). Analytical grade ammonium acetate and acetic acid were purchased
from Beijing Chemical Reagents Company (Beijng, China). Stock standard solution of
2000mg/L PHMB was prepared by dilution of 1mL PHMB standard with 100mL 0.01mol/L HCl and
stored at 4ºC. Working solutions (100, 200, 400, 800, 1000mg/L) were prepared by
serial dilution of the stock solution with 10mmol/L HCl and stored at 4ºC.
2.2 Apparatus
A Waters 2695-996 high performance liquid chromatography (Milford, MA, USA) was
employed with a Waters Empower Chromatography Manager Workstation for instrument control
as well as data acquisition and processing. The chromatographic procedure employed was
isocratic with a mobile of acetonitrile-20mmol/L ammonium acetate acidified with acetic
acid, pH 4.0 (16:84, v/v). The column used was Shiseido C18 (150×4.6mm, 5mm) (Shiseido Co. Ltd. Tokyo, Japan).
Chromatography was carried out with the column at 30ºC. The flow rate was 1mL/min with
injection volume of 20m L. UV chromatograms were extracted at a wavelength of 235nm.
PB303-S balance was purchased from Mettler -Toledo Instruments
Co. (Switzerland). JC-420pH/mV meter was purchased from Beijing Chuangye Instrumental
Plant (Beijing, China).
3 RESULTS AND DISCUSSION
3.1 Choice of detection wavelength
In general, the absorbance detection is the first choice in HPLC analysis of
PHMB owing to its simplicity and reliability. 20mL 200mg/L PHMB was directly injected into the HPLC-system without
column and scanned from 200 to 400 nm. The ultraviolet absorption spectrum of PHMB in
chromatographic mobile phase is illustrated in Fig.2. The maximum absorption is 235.7nm.
Therefore, the detection wavelength was selected at 235nm.
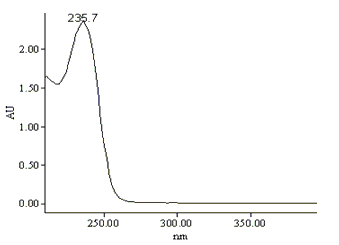
Fig.2 The ultraviolet absorption spectrum of PHMB in
chromatographic mobile phase. Chromatographic conditions are shown in section 2.2.
3.2 Choice of chromatographic
separation conditions
In general, PHMB exists in cationic form in aqueous solution [2]. So,
an anion-pair agent was added to form neutral ion-pairs to be retained by the C18 stationary
phase. To further improve the retention behavior of the ion-pair, inorganic salts such as
formate, phosphate and acetate were also added to produce a salting-out effect,
respectively. The experiment showed that acetate was a suitable ion-pair agent which
results in a good peak shape of PHMB.
The concentration of acetate in the mobile phase must be high (![]() 10mmol/L) enough
to provide good buffer capacity. However, higher concentration will result in potential
corrosive to pump seals. When 16% acetonitrile and the pH 4.0 of the mobile phase were
kept unchanged, five concentration levels of NH4AC at 12, 16, 20, 24 and
28mmol/L were added to the aqueous portion of the mobile phase, respectively. There were
no significant effects on both the retention time and peak area of PHMB. As a compromise,
20mmol/L was the best choice.
10mmol/L) enough
to provide good buffer capacity. However, higher concentration will result in potential
corrosive to pump seals. When 16% acetonitrile and the pH 4.0 of the mobile phase were
kept unchanged, five concentration levels of NH4AC at 12, 16, 20, 24 and
28mmol/L were added to the aqueous portion of the mobile phase, respectively. There were
no significant effects on both the retention time and peak area of PHMB. As a compromise,
20mmol/L was the best choice.
Acetonitrile is preferred to methanol in frequently used RP-HPLC
because of its relatively higher elution strength as well as the lower column back
pressure it creates. With the increase in acetonitrile concentration, the retention time
of PHMB is reduced when 20mmol/L NH4AC (pH = 4.0) remains unchanged in the
mobile phase. Taking into the separation efficiency and time, the concentration of
acetonitrile was set at 16%.
The pH of the mobile phase is one of the key factors affecting
both the retention behavior and peak shape of ionic analytes. When 16% acetonitrile and
20mmol/L NH4AC was kept unchanged, better peak shape of PHMB could be obtained
at pH 4.0 compared with pH 4.5 and 5.0, respectively. So, pH = 4.0 of the mobile phase was
chosen.
Under the above optimized conditions, the chromatogram of PHMB
standard solution is shown in Fig.3.
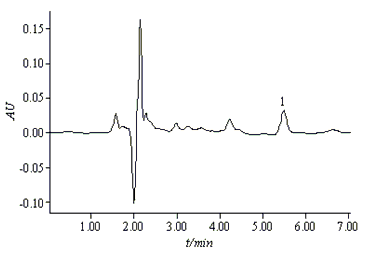
Fig.3 Chromatogram of PHMB (200mg/L) in a standard solution
Chromatographic conditions are shown in section 2.2
[1. PHMB (t = 5.5min)]
It was shown that PHMB
is well separated and eluted at a reasonable time. The retention factors (k) for PHMB
under isocratic separation conditions can be calculated as [3]
k = (tR – t0)/t0
(1)
Where tR and t0 are the retention time of
PHMB and the column dead time, respectively. The column dead volume for a 4.6 mm i.d.
column can be estimated as
VM![]() 0.1 L
(2)
0.1 L
(2)
Where VM is the column dead volume in milliliters and
L is the column length in centimeters. So, for current column, L is 150 mm or 15 cm, and
0.1×15 = 1.5mL. Convert VM to t0 by dividing the volume by the flow
rate: 1.5mL/1.0mL/min = 1.5min. k value for PHMB is 2.7, which falls into the ideal range
(2< k< 10) for separation.
The theoretical plate height, H, is obtained from the
chromatogram by:
H = (L /16) (Wt / tR) 2
(3)
Where L is the column length; Wt is the peak width at
the baseline (in seconds); and tR is the retention time (in seconds). The
calculated H for PHMB was 0.046mm and the theoretical plate number is 3191.
3.3 Detection limit, precision and
linear range
Under the optimized chromatographic conditions, 20mL of each PHMB working solutions was injected. The peak area (A)
and the concentration (C, mg/L) of PHMB had good linear relationship. The regression
equation was A = 0.0006C + 19.328 with a correlation coefficient r = 0.9997. The linear
range was from 100 to 1000 mg/L. The detection limit (S/N = 3) was 50mg/L. The precision
was evaluated by performing seven replicate analysis of a standard concentration of
200mg/L. The relative standard deviation for retention time and peak area were 0.7% and
1.2%, respectively.
3.4 Real sample analysis
3.4.1 Real sample determination
Three samples collected from the markets were diluted with ultra-pure water and
determined in triplicates after filtered through a 0.45 mm filter membrane. The results were 0.21, 0.20 and 0.22% (w/v),
respectively (see Table 1). Much attention should be paid that several drops of
acetonitrile must be added to eliminate the foaming formed in the process of dilution.
Table 1 Analysis of real samples
Sample |
Determined |
Specified |
RSD (n = 3) |
1# |
0.21 |
0.20 |
2.6 |
2# |
0.20 |
0.20 |
1.0 |
3# |
0.22 |
0.20 |
4.3 |
3.4.2 Recovery
Spike studies were performed by sample 1#, Three levels of PHMB were
added (100, 200, 400mg/L) after its dilution 1:10 with ultra-pure water. Recoveries are in
the range from 92.92 to 102.4% (see Table 2).
Table 2 Average recovery and relative standard deviations (RSD)
Determined |
Spiked |
Founded |
Recovery |
RSD (n = 3) |
212 |
100 |
310 |
99.06 |
1.3 |
200 |
417 |
102.4 |
0.9 |
|
400 |
597 |
92.92 |
0.9 |
4. CONCLUSION
The present method is simple, rapid, accurate and suitable for the routine assay of
PHMB in compound chemical disinfectants. However, there still exist problems when
analyzing PHMB at low levels (0.0001% (w/v)) in personal care products such as ophthalmic
solutions due to the high detection limit. This could be resolved by using a larger
injection volume as well as sample pre-concentrating and cleaning. The research is in
progress in our laboratory.
REFERENCES
[1] The Ministry of Public Health, Technical Standards for Disinfection, 2002
edition, Beijng: The Ministry of Public Health, 2002.
[2] Lu L X, Wei L F, Fu G M, et al. Chinese Journal of Disinfection (Zhongguo
Xiaoduxue Zazhi), 2005, 22 (1): 30-33.
[3] Dolan J W, LC-GC North America, 2004, 22(6): 524-528.
反相高效液相色谱法快速测定复方化学消毒剂中聚胺丙基双胍
车宜平1,2,丁晓静2,赵珊2,刘芃岩1
(1河北大学化学与环境科学学院,保定
071002;2北京市疾病预防控制中心,北京
100013)
摘要
本文建立了反相高效液相色谱法快速测定复方化学消毒剂中聚胺丙基双胍(PHMB)的新方法。以乙腈:20mmol/L醋酸铵
(冰乙酸调pH至4.0) = 16:84为流动相,Shiseido
C18 (150mm × 4.6mm, 5μm)柱为分离柱,柱温30℃,235nm处检测,7min内测定了样品中的PHMB。方法检出限:50mg/L (S/N = 3),精密度RSD
= 1.8% (n = 7),线性范围:100 ~ 1000 mg/L,低、中、高三种浓度水平的加标回收率在92.92% ~ 102.4%间。方法简单、快速,适于复方化学消毒剂中PHMB的测定。
关键词 反相高效液相色谱法;
聚胺丙基双胍;复方化学消毒剂