http://www.chemistrymag.org/cji/2009/113013pe.htm |
Mar.1,
2009 Vol.11 No.3 P.13 Copyright |
Chen Tieshi, Li Yongming, Zhao Yuping, Yang
Jun, Zhang Yuanming, Tang Yu
(Department of Chemistry, Jinan University, Guangzhou 510632, China)
Keyword selenium, nano-needle, surfactant, reverse microemulsion 1. INTRODUCTION
With a relatively low melting point (~490 K), high photoconducting (~8×104 S cm-1), and low photomelting temperature (~77K),elemental selenium has been widely applied in the field of rectifiers, solar cells, photographic exposure meters, and xerography[1, 2]. It is now commonly accepted that in order to exert ultimate performance and applications of inorganic materials with nano- and microscale, a key factor is to control their shapes and sizes. Many efforts have given some good results in synthesis of elemental selenium with specific shapes and sizes. The reported morphologies of selenium include nanotube[3], single-crystalline Se nanotube[4], submicrotube[5], microtube[6], nanorod[7-9], microrod[6, 10], nanobelt and nanowire[9, 11], nanoneedle[12], and so on. Compared with these successful works, special shapes of selenium, such as feathershape[13] and rose-like Se crystal[14], are seldom observed and difficult to be formed. Diversified methods, such as hydrothermal routes[3, 6-8], template inducing with surfactants[4-5, 11], small molecule-controlled process[10], have been applied to synthesize selenium. Of them, surfactants are effective soft template and used widely. In our previous works, multiple different morphologies of calcium carbonate were formed in the reverse microemulsion of Tx-100-SDS surfactants system[15]. In order to further understand the role of surfactants in the process of crystal growth and challenge the special morphology of selenium, the synthesis of selenium was carried out in reverse microemulsion solution of different surfactants in this paper. 2. EXPERIMENTAL
2.1 Materials
Polyoxyethylene nonyl phenyl ether(OP-10), Sorbitan monolaurate(Span-60), p-octyl polyethylene glycol phenyl ether(Tx-100), and tetradecyl dimetyl benzyl ammonium chloride (TDMBAC) were of chemically pure. Sodium dodecyl sulfate(SDS),cetyltrimethylammonium bromide(CTMAB) and sodium dodecyl benzene sulfonate(SDBS)and the other reagents were of analytically pure. All chemicals were used without further purification. Double distilled water was employed to prepare aqueous solution.
2.2 Preparation of nano-selenium in reverse micelle solution
The clear reverse micemulsion solutions A or B were prepared by adding 1ml of 0.25M aqueous solution of Na2SeO3 and nonionic surfactants, or 1.0mL of 1.0M aqueous solution of ascorbic acid and surfactant ionic surfactants into 15.5mL of cyclohexane and 2.5mL of n-butanol, respectively. After solution A had been dropped slowly into solution B under stirring at room temperature, the container of solution A was washed by 2mL of cyclohexane. Then this cyclohexane was added to the mixture of solution A and B also. The solution was kept to stir for 3-5mins, and then aged at room temperature for 48 hours. The collected precipitates were washed with acetone, distilled water and ethanol, respectively, and then dried at room temperature.
2.3 Characterization
The resulting precipitates of selenium were characterized by SEM (JEOL JSM-T300). The X-ray diffraction (XRD) measurement was conducted by MSAL XD-2 powder X-ray diffractometer using Cu Ka radiation of wavelength 1.5406 Å at 40 kV and 20 mA). 3. RESULTS AND DISSCUSSION
3.1 Influence of SDS/Tx-100 concentration on the morphology of nano-selenium
The SEM image (Fig.1a) shows the aggregation of rod-like selenium particles at Tx-100-SDS concentration of 0.05M. Submicron selenium rod with the length ca. 10-15um and diameter ca. 100-300nm could be seen in figure 1b when the surfactant concentration was increased to 0.15M. When the surfactant concentrations are higher to 0.25M or 0.35M, the resulting nano-selenium needles with sharp tips(fig c, length ca. 3-10um and fig d, length ca. 2-8um) aggregate to form the sphere-like morphology. It is more difficult to prepare the nanoneedles because at present few methods are designed to successfully prepare the nanoneedles with sharp tips[12]. Furthermore, the formed morphology by nanoneedles is also difficult to be achieved in seldom reported special morphologies[13,14].

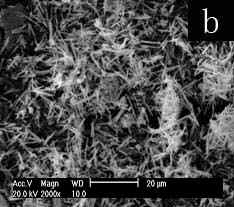

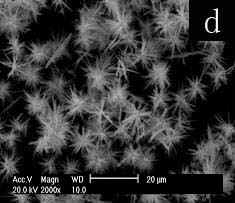
Fig.1 SEM images of selenium
paticles prepared at different concentrations of Tx-100-SDS (total concentration of SDS
and Tx-100 at molar ratio of 1:1): (a) 0.05M, (b) 0.15M, (c) 0.25M, (d) 0.35 M.
Obviously, the higher concentrations of Tx-100-SDS help to disperse the selenium
particles, diminish the size to nano-scale, and form sphere-like morphology of needle
congeries. Thus, the results display the important role of Tx-100-SDS concentration in
controlling the size and morphology of selenium.
Fig.2 is XRD
pattern of nano-selenium prepared at 0.25M concentration of Tx-100-SDS. The sharp
diffraction peaks indicate the good crystallization of the products. Those peaks of (100),
(101), (110), (012), (200), (201), (003), (013) and (120) were clearly observed, which are
right consistent with the values of the hexagonal phase of Se crystal structures(JCPDS
File NO 86-2246).
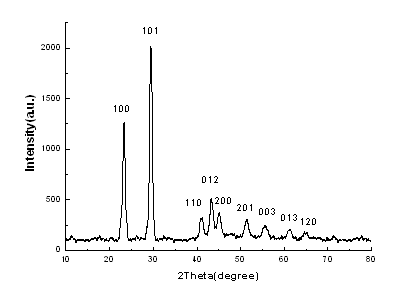
Fig.2 XRD pattern of nano-selenium prepared
at 0.25M concentration of Tx-100-SDS.

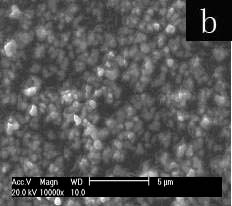
Fig.3 SEM images of selenium
particles prepared at 0.25M concentration of SDS(a) or Tx-100(b).
At 0.25M, the concentration of Tx-100-SDS to form the sphere-like nano-selenium of needle
congeries, the only SDS or Tx-100 results in aggregation of amorphous selenium particles
(Fig. 3). The result points out the interaction of SDS with Tx-100 influences the
morphology of nano-selenium very much.




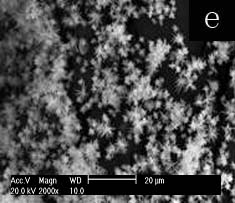
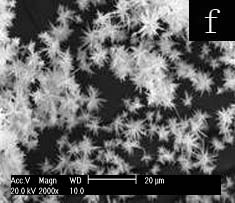
Fig.4 SEM images of selenium particles prepared at 0.25M concentration other mixed surfactants for 48h: (a) OP-10-SDS, (b) Span60-SDS, (c) Tween20-SDS, (d) Tx-100-SDBS, (e) Tx-100-CTMAB, (f) Tx-100-TDMBAC
To understand the influence of interaction between SDS and Tx-100 on the morphology of selenium, the other surfactants were employed at the concentration of 0.25M. Firstly, Tx-100 was replaced by other nonionic surfactants, OP-10, Span60 and Tween20, respectively. Tween20 couldn’t dissolve in the reverse micelle solution at 0.25M of Tween20-SDS, thus the concentration was changed to 0.015M. OP-10(Fig. 4a) and Tween20(Fig. 4c) result in dispersed rod like selenium, whereas SP60(Fig. 4b) gives aggregation of amorphous selenium particles. The result could be ascribed to OP-10 and Tween20 bear the similar EO chain with Tx-100 and can dissolve in water, but SP60 is totally different with Tx-100 in the structure and solubility in water. The results reflect that Tx-100 plays an important role in the effect on morphology of selenium. Then SDS was replaced by other ionic surfactants, SDBS, CTMAB and TDMBAC, respectively. Correspondingly, the SEM images show the nano-selenium of needle congeries as cotton-like for SDBS(Fig. 4d, length ca. 7-20um), sphere-like for CTMAB(Fig. 4e length ca. 1-2um,) and TDMBAC(Fig. 4f, length ca. 3-7um). Unlike replacement of Tx-100, ionic surfactants with different structure cause small morphology variation of selenium. Selenium is formed by reducing Na2SeO3 with ascorbic acid. As negative ion, SeO32- tends to get close to cationic surfactant and far from anionic surfactant. Thus the effects of anionic surfactant on SeO32- would be diminished by electrostatic repulsion. In aqueous solution, the ionic heads of surfactants point out to water in micelles. So, if selenium is formed by reducing SeO32- in water instead of in micelles, different results should be expected for anionic and cationic surfactants. But it is not the fact. On the other hand, the non-polar heads of different surfactants form the similar circumstance of micelle cores. Thus, it is reasonable to speculate on selenium particles are formed by reducing SeO32- with ascorbic acid existing in micelles cores for the sake of like structure with non-polar heads of surfactants. The formation of selenium in micelle will be in favor of surfactants to template the growth of selenium particles.
4. CONCLUSIONSAt the 0.25M concentration of Tx-100-SDS, the prepared nano-selenium needles with sharp tips aggregate to form the sphere-like morphology. Tx-100 is important to prepare selenium crystals with special morphology, while anionic SDBS and cationic CTMAB or TDMBAC give the similar effects on the crystal growth of selenium. Possible mechanism is proposed that selenium particles are formed by reducing SeO32- with ascorbic acid existing in micelles cores for the sake of like structure with non-polar heads of surfactants, and templated to grow by surfactants.
[1]Berger L. I. Semiconductor Materials; CRC
Press: Boca Raton, FL, 1997.
[2]Johnson J. A.; Saboungi M. L.; Thiyagarajan P.; etal,. J. Phys. Chem. B 1999, 103: 59.
[3]Xi G Ch; Xiong K; Zhao Q B; etal, Cryst.
Growth Des., 2006, 6(2): 577-582.
[4]Zhang Sh Y; Zhang J; Liu Y; etal, Electrochimica Acta
2005, 50: 4365–4370.
[5]Bin Zhang, Wei Dai, Xingchen Ye, Weiyi
Hou, Yi Xie, J. Phys. Chem. B 2005, 109: 22830-22835.
[6]Fan H; Wang Zh H; Liu X Zh; etal, Solid State
Communications 2005, 135: 319–322.
[7]Chen Y T; Zhang W; Fan Y Q; etal, Materials Chemistry and Physics 2006, 98: 191–194.
[8]Carter S. A.; Scott J. C.; Brock P. J., Appl. Phys. Lett. 1997, 71: 1145.
[9]Gao F; Lu Q Y; Meng X K; etal, J Mater Sci. 2008, 43: 2377–2386.
[10]Kamalesh M.; Poulomi R.; Suneel K. S.,
Cryst. Growth Des., 2008, 8(5): 1580-1584.
[11]Ma Y R; Qi L M; Shen W; etal, Langmuir 2005, 21:
6161-6164.
[12]Xiong Sh L; Xi B J; Wang W Zh;
etal, Cryst. Growth Des.,2006, 6(7): 1711-1716.
[13]Batabyal S.K.; Basu C.; Das A.R.; etal, Cryst.
Res. Technol. 2006, 41(7): 653 – 657.
[14]Deng D W; Yu J Sh;Pan Y,Eur. J. Inorg. Chem. 2008, 1129–1134.
[15]Tang Y; Du B Y; Li L G; etal, Chin. Sci. Bull.,2007, 52: 78-83.
陈铁石,黎永铭,赵玉苹,杨 骏,张渊明,唐 渝
(暨南大学化学系,广州 510632)
摘要 在表面活性剂Tx-100-SDS形成的反相微乳液中,室温下用抗坏血酸还原Na2SeO3简便地制备了硒的纳米晶体,在0.25M的表面活性剂浓度下,具有锐利针尖的纳米针硒晶体聚集形成了球形,用OP-10、Tween20或SP60代替Tx-100导致硒失去了特殊形貌,其他阴离子的SDBS、阳离子的CTMAB或TDMBAC与Tx-100混合都得到了与SDS类似的具有特殊形貌的纳米硒晶体,Tx-100以及其与SDS的共同作用是制备具有锐利针尖的纳米针硒晶的体球形聚集关键因素,提出了可能机理。
关键词 硒,纳米针,表面活性剂,反相微乳液